
At the International Center for Spinal Cord Injury at Kennedy Krieger Institute, a 36-year old male with paraplegia secondary to intramedullary hemiangioblastoma works on locomotor training during a therapy session with a robotic assist.
By Dennis Tom-Wigfield, PT; DPT, Erin Haynes, SPT; and Rebecca Martin, OTR/L, OTD
In the United States, more than 7 million individuals have a form of paralysis. These persons identify themselves as having significant difficulty performing daily tasks due to a neurological insult to their body. Paralysis can be caused by stroke, post-polio syndrome, cerebral palsy, multiple sclerosis, traumatic brain injury, or spinal cord injury. These individuals have a lifestyle which operates differently from their peers.1 In addition to loss of motor and sensory function, paralysis negatively affects bone density, significantly decreases muscle mass, and impairs the cardiovascular system. Many neurological injuries also cause disruption to the sympathetic nervous system as well, which results in an increased risk for autonomic dysreflexia, slow and irregular heart rhythms, and sudden changes in blood pressure.
The International Center for Spinal Cord Injury (ICSCI) at Kennedy Krieger Institute in Baltimore is a leader in innovative rehabilitation approaches for people affected by spinal cord injuries. Utilizing Activity Based Restorative Therapy (ABRT), ICSCI aims to offset the chronic complications associated with spinal cord injury (SCI) and optimize the nervous system for recovery. The basis for ABRT is “repetitive-task specific training using weight bearing activities and external facilitation of neuromuscular activation.”2 Therapy sessions incorporate massed practice, weight bearing activities, functional electrical stimulation, task-specific practice, and locomotor training for up to 5 hours daily with specific considerations to perform near-normal kinematics and conditions. By incorporating advanced technology, ICSCI aims to offer a wider range of patients the opportunity to participate in near-normal motor activities.
BENEFITS, CONTRAINDICATIONS
Robotic gait training has the potential to maximize treatment efficiency and to improve functional outcomes in individuals with neurological injuries. It has been shown to facilitate improved walking speed, trunk and lower extremity strength, range of motion, balance, and blood circulation. Benefits also include more independence with ambulation and decreases in muscle spasms. Restorative Therapies’ RT600, RehaTechnology’s G-EO Evolution, and Bionics’ Ekso are robotic gait trainers that ICSCI implements. Each of these devices has unique functions to offer patients and their treating therapists. All offer customizable set-up to obtain high levels of repetitive stepping, while decreasing staff burden and potential for human error with manual cues. The gait trainers provide multiple options for adjustments, including partial body weight support, passive or active movement, controlled step length, electrical stimulation, and ambulation speed. With a variety of robotic equipment available, it is important for physical therapists to understand the opportunities and limitations of each device to determine what is most appropriate for their patients.
A wide variety of patients are appropriate for robotic gait training. However, it is important to be aware of the contraindications. Absolute contraindications include severe orthostatic hypotension, significant cardiac disease, vascular disease, integumentary issues such as open wounds, or pregnancy. Precaution should be taken if a patient has significantly declined bone density as indicated by dual-energy X-ray absorptiometry (DXA) or a history of pathological fractures to minimize risk of fractures. Each robotic device has specific parameters required for adequate lower extremity range of motion, tolerance to standing, and muscle strength. During evaluation, the therapist should recognize the patient’s emotional and behavioral response to robotic interventions as well.
WHAT NEUROLOGICAL OUTCOMES SHOW
Robotic gait devices show great promise in improving functional outcomes for patients with neurological injury. The Shin et al, 2014 study compared robotic-assistive gait training to over-ground gait training for 60 patients with incomplete spinal cord injury by conducting 10 treatments over 5 days. Both groups demonstrated comparable improvements in lower extremity function, ambulation, and mobility, and increases in range of motion and ankle muscular activation. The robotic-assistive gait training group demonstrated greater independence with walking.
Robotic gait training studies are limited with the SCI population. However, much research has been conducted on stroke rehabilitation and robotics. Mehrholz et al’s 2013 systematic review determined the use of robotic gait training for patients less than 3 months post-stroke were more likely to regain independent walking and improve gait velocity than subjects who received standard gait training. Hesse et al in 2012 conducted a study of 30 non-ambulatory patients with acute strokes. The results demonstrated remarkable gains with robotic gait training, as shown by improvements on ambulation outcome measures with significantly improved indicating improved functional ambulation. This current evidence suggests that if robotic gait training is utilized in concert with standard physical therapy early on after stroke, then initial non-ambulatory patients may achieve increased levels of independence with ambulation.
TESTING THE TECHNOLOGIES
RT600 is a functional electrical stimulation (FES) elliptical ergometer. This device has a motorized upright ergometer with a 12-channel electrical stimulator. Typically, the erector spinae, gluteals, quadriceps, hamstrings, anterior tibialis, and gastrocnemius are stimulated to increase neuromuscular activation. RT600 provides partial body weight support through its harness, has adjustable weight parameters, and allows for the patient to passively or actively participate in the elliptical stepping motion. Patients must be able to partially support their body weight with lower extremity muscle activation to use the machine properly to prevent a crouched posture. This can be achieved from electrical stimulation or volitional movement. RT600 requires significant set-up time because of skilled harnessing and 12 leads of electrical stimulation. Patients must be able to tolerate lower extremity electrical stimulation and 45 to 60 minutes of supported standing for optimal benefits.4
Hamzaid et al’s, 2012 study of five chronic spinal cord injury patients performed both electrical stimulation ergometer elliptical stepping and seated cycling. This study concluded the patients had increased power output during ergometer elliptical stepping, which indicated increased lower-extremity activation. This result suggests there could be increased benefits of upright positioning, functional electrical stimulation, and weight-bearing that the RT600 provides.
The G-EO Evolution is a body weight supported, end effector gait training device. Once harnessed, patients are set up in the machine by standing on two mobile force plates. End-effector devices use ground reaction forces to adjust and create a near-normal gait pattern by contact only at the patient’s foot and ankle. This set-up enables the patient to engage in the entire gait cycle to their maximal potential. G-EO Evolution has both a level stepping and stair climbing mode that can be utilized in passive, active-assistance (adaptive), or active mode. Adjustments can be made for body weight support, step cadence, velocity, step length, step height, and foot angle. G-EO Evolution includes an upper extremity bar and posterior and lateral hip supports. G-EO Evolution is indicated for patients with flaccid or active lower extremities and allows for adult and pediatric set-up. Passive mode can be used to increase blood circulation and promote trunk and lower-extremity muscle activation on individuals with flaccid lower extremities. The set-up time for the G-EO is reported to be low enough to reduce the therapist burden and improve session efficiency.
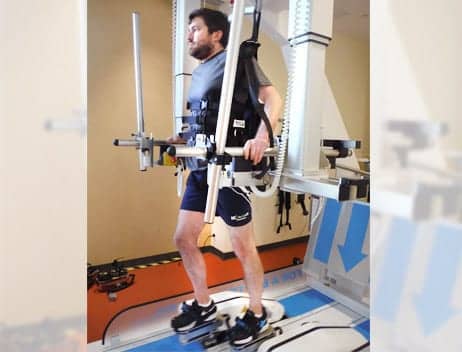
Study subject using a body weight supported, end effector gait training device. Over the course of therapy, the individual improved scores on the BERG balance test, 6 Minute Walk Test, and 10M Walk Test.
Tomelleri et al’s, 2011 research study compared ground reaction forces of patients during over-ground ambulation, walking on a treadmill, and on the G-EO in active and passive modes. This study indicated G-EO Evolution’s adaptive mode had comparable ground reaction forces to over-ground ambulation, indicating that patients experience proprioceptive feedback and extroceptive sensation similar to actual floor walking. Hesse et al’s, 2004 study with four SCI patients each received 5 weeks of ambulation on G-EO for 25 minutes a day with electrical stimulation. The subjects demonstrated increased gait speed, endurance, lower-extremity activation, and ability with over-ground ambulation.
Bionics’ Ekso exoskeleton also falls into the robotic category, but is the only device reviewed here that provides over-ground ambulation. The structure of Ekso supports itself and a patient’s weight, allowing them to experience full weight bearing, regardless of strength. It provides 0% to 100% assistance on each leg as needed, and the assistance levels can be adjusted independently of one another by the therapist. Ekso has multiple modes, allowing for the therapist or patient to initiate steps in different ways. Patients at ICSCI have demonstrated interest in Ekso due to potential for community ambulation and recognizing a more familiar walking pattern. Ekso is indicated for patients with a lower-extremity weakness due to stroke, spinal cord injury, and most neurological conditions. Ekso is appropriate for patients with complete and incomplete spinal cord injuries from C7 and below. Ekso requires either bilateral upper-extremity strength or one functional upper extremity and one functional lower extremity. Patient must be able to be transferred without a harness or lift to the chair that the Ekso rests in during setup.
Ekso has been demonstrated to improve walking speed, increase distance, recover lower extremity muscle firing sequence, and decrease gait deviations. Kessler Foundation has indicated that SCI patients utilizing Ekso have demonstrated “increased step swing time, stride length, hip extension, and muscle activation more similar to normal gait cycle” after a couple of training sessions.16 Louie et al’s, 2015 performed a systematic review and correlational study of non-ambulatory spinal cord injury patients who had physical therapy that included massed practice with powered exoskeletons. The review concluded that exoskeletons can improve gait speeds for non-ambulatory patients with thoracic-level motor-complete SCI; increased gait speed is correlated to the patient’s level of injury and amount of practice in the exoskeleton.
CASE STUDIES
Patients at ICSCI involved in robotic gait training have shown promising results. The following are three case studies that illustrate device selection, limitations, and commensurate results.
Patient A is a 36-year old male who was diagnosed with paraplegia secondary to intramedullary hemiangioblastoma and significant syrinx with subsequent removal in 2013 and 2015. He was diagnosed with T4 American Spinal Injury Association (AIS) D after his second surgical removal of the tumor in 2015. He presented to ICSCI ambulating with two lofstrand crutches and a Bioness on his right ankle. He participated in physical therapy for 2-hour sessions, twice a week, for 4 months. Patient A underwent many interventions including G-EO gait training, locomotor training (LT) on the therastride, gait training on a split belt treadmill, trunk and lower-extremity strengthening and balance exercises. He participated in six 20- to 30-minute G-EO sessions during treatment. He began in active-assist mode with 5% to 15% assistance, 20-40 pounds of BWS, and achieved 1200 steps in 30 minutes.
By the last session, he was able to achieve 2000 steps during a 30-minute session with no assistance and up to 10% resistance in active mode. Per the patient report, he preferred training on the G-EO over other body weight support devices due to the increased comfort of the harness and the smoothness of the gait cycle. Over the course of therapy, Patient A improved his scores on the BERG balance test, the 6 Minute Walk Test, and the 10M Walk Test. Furthermore, Patient A progressed to full-time ambulatory with a single point cane and the Bioness in the community and to no assistive device and the Bioness within his home. Robotic gait training in combination with ABRT allowed Patient A to progress to ambulation with a lesser assistive device, improved his dynamic standing balance, and improved his walking speed.

Therapist works with a 30-year old female diagnosed with tetraplegia and brain injury secondary to a motor vehicle accident.
Patient B is a 30-year old female diagnosed with tetraplegia and brain injury secondary to a motor vehicle accident 14 years ago. She is diagnosed with C6 AIS C. At initial evaluation she ambulated with a platform walker, bilateral knee KAFOs with an adduction bar, TLSO and maximal assistance x 2. External assistance was provided to promote hip and trunk flexion, to control the walker, and to assist with foot placement at initial contact. She was able to ambulate 76 feet during a 6 Minute Walk Test on initial evaluation. G-EO was implemented for Patient B to promote lower-extremity activation and increase trunk control.
Patient B participated in three 24- to 45-minute sessions on the G-EO along with over-ground gait training with braces, body weight support gait training with no braces, bed mobility, and seated core strengthening exercises. The EKSO gait trainer was not used due to contractures and weakness in (B) hands and arms. Over multiple bouts she was able to achieve more steps on the G-EO with decreased assistance. Per the patient report, she felt contractions in her right hamstring while on the G-EO, which were palpable to the therapist. This was the first contraction she felt in her hamstrings in years. During her re-evaluation she ambulated 126 feet on the 6 Minute Walk Test with setup as above and with decreased external support. She also improved her independence with bed mobility and her seated functional reach scores. Patient B demonstrated an improvement with gait as well as trunk strength over her course of care.
Patient C is a 52-year-old male diagnosed with T6 AIS C paraplegia secondary to traumatic stabbing 10 years ago. At initial evaluation, he presented as non-ambulatory with severe hypertonicity in his lower extremities, decreased core strength, and was dependent on his manual wheelchair for all mobility. He utilized all three robotic gait trainers to decrease hypertonicity, improve core strength, and to promote lower-extremity neuromuscular activation and recovery. Other interventions included advanced wheelchair skills training, vibration plate, seated core strengthening, and seated balance training. With the EKSO, he was able to ambulate up to 120 steps during each 45- to 60-minute session. He participated in three EKSO gait training sessions total.
The amount of assistance decreased from maximal to minimal for hitting his lateral and forward weight shift targets over the three sessions. During the RT600, Patient C was able to increase his bilateral weight bearing from 30% to a consistent 50% over three 60-minute sessions. On the G-EO, he ambulated on both passive and active-assistive mode for up to 45 minutes each time. Patient C’s hypertonicity was a limitation on all three devices; the tone decreased step length, number of steps taken, and gait speed. He demonstrated decreased muscle spasms as interventions progressed on all three devices. Patient C indicated significant psychosocial benefit from his robotic gait training interventions as well; he frequently stated he enjoyed being upright and getting the opportunity to move his legs. This patient was not appropriate for a functional ambulation goal during this episode of care. However, he demonstrated increased independence with over-ground ambulation with partial body weight support and external assistance from the therapist. In concert with robotic gait training and standard physical therapy interventions, this patient met all his functional goals, which included improved lateral reach, trunk control, lower extremity tone reduction, and success with advanced wheelchair skills.
Each of the three patients present with a varying degree of paralysis; one with tetraplegia dependent on a power chair for mobility, one with paraplegia dependent on a manual chair for mobility, and one with paraplegia that is full-time ambulatory. Despite their varying functional levels, all were appropriate to trial robotic gait training. Each patient used the robotic gait trainers for different reasons, whether it was to increase independence with ambulation, improve core strength, or decrease spasticity. The functional outcome measures for each of the patients indicated progress toward functional goals, including an increase in independence with ambulation in one case. It should also be noted that each of the patients demonstrated positive psychosocial response to robotic gait training. Robotic gait training demonstrates a promising future for making functional improvements in patients with spinal cord and other neurological injuries. More research is needed to better understand the implications of robotic gait training for patients with acute and chronic spinal cord injuries, as well as for the ambulatory and non-ambulatory populations. Robotic gait trainers will continue to be clinically assessed at ICSCI in conjunction with ABRT in hopes of fully unlocking our patients’ potential for recovery. RM
Dennis Tom-Wigfield, PT, DPT, CRND, has been a physical therapist at Kennedy Krieger Institute since 2013 and holds a certification in Rare and Neuroimmunologic Disorders. He attended physical therapy school at the University of Maryland, Baltimore, and since graduation has focused his work on spinal cord injuries.
Erin Haynes, SPT, is a third-year student at the University of Mississippi. When this article was written in 2016, Haynes was on her third clinical rotation at Kennedy Krieger Institute.
Rebecca Martin, OTR/L, OTD, is the manager of clinical education and training for the International Center for Spinal Cord Injury at the Kennedy Krieger Institute in Baltimore. For more information, contact [email protected].
References
- Christopher and Dana Reeve Foundation. One Degree of Seperation. 2009. Accessed February 22, 2016.
- Dolbow D, Gorgey A, Recio A, et al. Activity-based restorative therapies after spinal cord injury: interinstitutional conceptions and perceptions. Aging and Disease (2015). 6(4) 254-261.
- Reha Technology. The G-EO System. Accessed February 13, 2016.
- Barriskill A. RT600 Summary of Safety and Effectiveness. Published April 4, 2011. Accessed March 5, 2016.
- Ekso Product Information. Updated 2015. Accessed February 22, 2016.
- Mehrholz J, Elsner B, Werner C, Kugler J, Pohl M. Electromechanical-assisted training for walking after stroke. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD006185. DOI: 10.1002/14651858.CD006185.pub3
- Hesse S, Tomelleri C, Bardeleben A, Werner C, Waldner A. Robot-assisted practice of gait and stair climbing in nonambulatory stroke patients. J Rehabil Res Dev. 2012; 49(4):613–22. DOI: 10.1682/JRRD.2011.08.0142
- Shin J, Kim J, Park H, Kim N. Effect of Robotic-assisted gait training in patients with incomplete spinal cord injury. Ann Rehabil Med. 2014;38(6):719-725. DOI: 10.5535/arm.2014.38.6.719
- Morawietz C, Moffat F. Effects of locomotor training after incomplete spinal cord injury: a systematic review. Physical Medicine and Rehabilitation. 2013 Nov;94(11):2297-2308. DOI: 10.1016/j.apmr.2013.06.023
- Reha Technology. G-EO System Technical Datasheet. Accessed February 13, 2016.
- Hamzaid, NA, Pithon, K, Smith, R, Davis, G. Functional electrical stimulation elliptical stepping versus cycling in spinal cord-injured individuals. Clinical Biomechanics. 2012;27:731–737.
- Tomelleri C, Waldner A, Werner C, Hesse S. Adaptive locomotion training on an end-effector gait robot evaluation of the ground reaction forces in different training. IEEE International Conference on Rehabilitation Robotics. 2011.
- Sale P, Franceschini M, Waldner A, Hesse S. Use of the robot assisted gait therapy in rehabilitation of patients with stroke and spinal cord injury. Eur J Phys Rehabil Med. 2012;48:111-21.
- Hesse S, Werner C, Bardeleben A. Electromechanical gait training with functional electrical stimulation: case studies in spinal cord injury. Spinal Cord. 2004;42:346-352.
- Forrest G et al, Kessler Foundation. The potential of the Ekso Exoskeleton for affecting long-term health and well-being in the SCI population. Presented at the Academy of Spinal Cord Injury Professionals (ASCIP) September 2012.
- Johnsen E, Ramanujam A, Garbarini E, Lamb R, Forrest G. Kessler Foundation. Adaptation of exoskeleton-assisted walking for non-ambulatory spinal cord injury: preliminary results. Presented at the World Congress of Biomechanics, July 2014.
- Louie D, Eng J, Lam T, and Spinal Cord Injury Research Evidence (SCIRE) Research Team. Gait speed using powered robotic exoskeletons after spinal cord injury; a systematic review and correlational study. J Neuroeng Rehabil. (2015) 12:82 doi 10.1186/s12984-015-0074-9.




