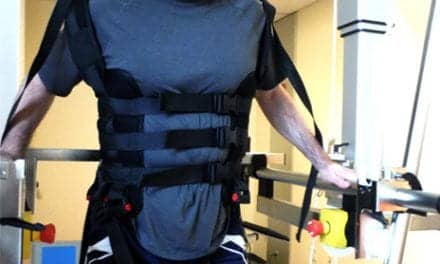
Ekso Bionics Indego Therapy with FES offers ten channels of electrical stimulation, which can be individually connected to a selection of muscle groups.
Ekso Bionics showcased the new Ekso Indego Therapy with Functional Electrical Stimulation (FES) at the American Physical Therapy Association’s Combined Sections Meeting in San Diego, Calif.
The company recently filed a Premarket Notification 510(k) with the US Food and Drug Administration that covers the addition of integrated FES hardware and software to Ekso Indego Therapy, a lower-limb powered exoskeleton recently acquired by Ekso.
Ekso Indego Therapy with FES offers ten channels of electrical stimulation, which can be individually connected to a selection of muscle groups, including quadriceps, hamstrings, gluteus maximus, tibialis anterior, gastrocnemius, trunk flexors and trunk extensors.
It automatically adapts the timing of stimulation to match a patient’s speed in motion+ and advanced gait programs, and each of the ten FES channels may be customized, allowing for individualized user settings and therapy programs.
“This unique integrated solution is a testament to Ekso Bionics’ continued commitment to innovation and delivering on our promise of maximizing patient outcomes,” said Scott Davis, chief executive officer of Ekso Bionics. “Individuals with spinal cord injury or stroke who use Ekso Indego Therapy in a rehabilitation setting will soon have the opportunity to benefit from FES in further improving limb function, balance and muscle strength.”
Ekso Bionics is a developer of exoskeleton solutions that support or enhance strength, endurance and mobility across medical and industrial applications.
The company offers technologies that range from helping those with paralysis to stand up and walk, to enhancing human capabilities on job sites.
Photo via Ekso Bionics