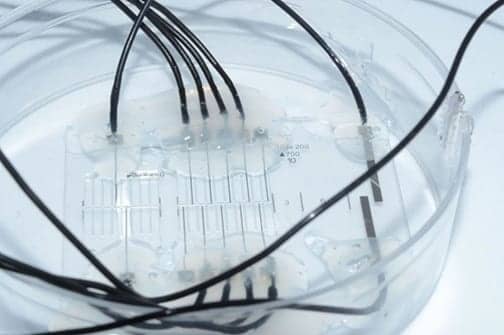
The “nerve-on-a-chip” platform, pictured here, is designed to stimulate and record peripheral nerve fibers on a chip. (Photo courtesy of EPFL.)
Scientists have developed a nerve-on-a-chip platform for the stimulation and recording of peripheral nerve fibers on a chip. This platform may pave the way to using chips to improve neuroprosthetic designs, they suggest.
The platform, developed by researchers at the lab run by Dr Stéphanie Lacour, a professor at Ecole Polytechnique Fédérale de Lausanne (EPFL’s) School of Engineering, is designed to stimulate and record from explanted nerve fibers, just as an implanted neuroprosthetic would. It contains microchannels embedded with electrodes and explanted nerve fibers to faithfully replicate the architecture, maturity and functioning of in vivo tissue.
The platform can be manufactured in a clean room in 2 days and is able to rapidly record hundreds of nerve responses with a high signal-to-noise ratio. However, according to the scientists in a media release, it can record the activity of individual nerve cells.
The scientists tested their platform on explanted nerve fibers from rats’ spinal cords, trying out various strategies for stimulating and inhibiting neural activity. They also used the platform to test a photothermic method for inhibiting neural activity.
Their research was published in Nature Communications.
“Neural inhibition could be a way to treat chronic pain like the phantom limb pain that appears after an arm or leg has been amputated, or neuropathic pain,” Lacour says in the release.
During their research, they deposited a photothermic semiconducting polymer, called P3HT:PCBM, on some of the chip’s electrodes.
“The polymer heats up when subject to light. Thanks to the sensitivity of our electrodes, we were able to measure a difference in activity between the various explanted nerve fibers. More specifically, the activity of the thinnest fibers was dominantly blocked,” says Sandra Gribi, a PhD student at the Bertarelli Foundation Chair in Neuroprosthetic Technology, the release continues.
“And it’s precisely those thin fibers that are nociceptors—the sensory neurons that cause pain. The next step will be to use the polymer in an implant placed around a nerve to study the inhibiting effect in vivo.”
The scientists also used their platform to improve the geometry and position of recording electrodes, in order to develop an implant that can regenerate peripheral nerves. By running the measured neural data through a robust algorithm, they will be able to calculate the speed and direction of nerve impulse propagation—and therefore determine whether a given impulse comes from a sensory or motor nerve, per the release.
“That will enable engineers to develop bidirectional, selective implants allowing for more natural control of artificial limbs such as prosthetic hands,” Lacour adds.
[Source(s): Ecole Polytechnique Fédérale de Lausanne, Science Daily]


