The development of pressure ulcers is a well-known problem that occurs in all settings and among all populations. Pressure ulcers are receiving increased attention with the recent implementation of the Centers for Medicare and Medicaid Services (CMS) Inpatient Prospective Payment System, where hospitals are no longer reimbursed for the treatment of hospital-acquired pressure ulcers that were not present on admission.1 It is important for health care providers in all settings to identify, treat, and, most importantly, aid in the prevention of pressure ulcers.
The National Pressure Ulcer Advisory Panel (NPUAP) defines pressure ulcers as a “localized injury to the skin and/or underlying tissue usually over a boney prominence, as a result of pressure, or pressure in combination with shear and/or friction.”2 The two most frequent anatomical places where pressure ulcers develop are over the boney prominences of the sacrum and heels.3 Since these areas are most vulnerable while lying on the back, patients with significant impairments in mobility are typically at greater risk for developing pressure ulcers. Therefore, a majority of prevention methods are focused at relieving pressure over these areas. However, in patients who have minimal impairment in mobility or who may otherwise not be at risk for developing a pressure ulcer, clinicians may neglect to recognize that medical devices, including orthoses, can also cause pressure ulcers even if they do not occur over boney prominences.
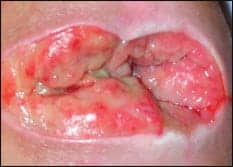 |
| Medical devices, such as orthoses, can cause pressure sores, like the one above. |
WOUNDS CAUSED BY MEDICAL DEVICES: ORTHOTICS
Medicare defines an orthosis as a device that is applied to the outside of the body that supports and aids in rehabilitation of existing body parts.4 Orthoses can be used to stabilize and/or immobilize parts of the body, prevent deformity, protect against injury, or assist with function. An orthosis can be applied to most parts of the body, including cranial, cervical, truncal, pelvic, hip, upper extremities, and lower extremities. Many times, the use of an orthosis is intended to prevent skin breakdown or offload an area from pressure such as in the heel. Often the orthosis itself can be the cause of a pressure ulcer, although the initial intention was prevention. It is the responsibility of the health care provider, including the therapist and nurse, to complete regular skin checks and ensure correct fit of the orthosis. In addition, education of the patient on appropriate use, fit, and prevention of orthosis-related pressure ulcers is required.
BEST PRACTICE IN PREVENTION
Patients with any device require excellent skin care and sensation to prevent breakdown. If applicable, the patient should be taught how to perform self-care with the orthosis so it may continue upon discharge if needed. Frequent skin checks are important to identify early signs of breakdown, including forces from pressure, friction, and shear. Regular cleansing of the skin is an integral facet of wound care prevention. If the area is not regularly cleansed and a wound develops, the patient is at risk for a skin infection in addition to the increased costs associated with hospital-acquired pressure ulcers. Skin cleansing can be accomplished through the use of soap and water or the use of medical grade products such as a no-rinse foam cleanser. The skin should be allowed to completely dry prior to the application of the orthosis. Excess moisture under a device can macerate the skin, causing breakdown as well as increasing the incidence of fungal infections.
The device itself should also be cleansed regularly. A good practice is to have two orthoses or padding inserts for rotation while one is being cleansed or the patient is being bathed. If the device is ill-fitting and early signs of breakdown are noted such as redness, blistering, or abrasions, prompt attention to adjust or replace the device is required to prevent further skin deterioration.
COMMONLY USED ORTHOSES
Cranial—Cranial orthoses typically include protective helmets or custom-made helmets. Their use is to protect the cranium such as after a hemicraniectomy. These devices are used when patients are out of bed and are typically removed only for skin assessments and to provide hygiene. The shape of the skull after surgery creates increased areas of pressure with off-the-shelf helmets rather than custom-made helmets. In addition, the straps that secure the helmet place pressure on the chin and ears, which may lead to skin breakdown.
Cervical/Cervicothoracic—Cervical collars can be rigid, semirigid, or soft. Both types of orthoses are intended to stabilize the cervical and thoracic spine after trauma, surgery, or degenerative disease. Cervical orthoses increase the risk for breakdown in areas such as the occiput, chin, sternum, shoulders, back, and body of the mandible.5 Skin damage associated with the use of cervical collars is directly related to the number of days worn.5,6 The more days the collar is worn, the greater the incidence of breakdown. Nonorthosis-specific indicators that predict breakdown include ICU admission, mechanical ventilation, necessity for cervical MRI, and time for spine clearance and removal of the collar.6 These studies also identify edema, poor fitting collars, matted hair, the presence of foreign bodies, and excessive moisture as contributors to pressure ulcer development. Several articles discuss preferences toward particular cervical orthoses; however, there are no rigorous and conclusive studies that support the use of one particular collar to prevent pressure ulcers over another.5,7,8
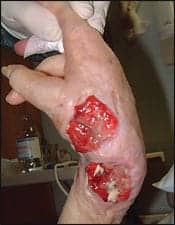 |
| Prolonged contact with any orthoses can be detrimental to skin integrity. |
Cervicothoracic orthoses may lead to skin breakdown in all of the aforementioned areas as well as the rib cage where the hard edges of the vest have prolonged contact with the skin. In addition, as the patient becomes more mobile, there is an increase in shearing and friction forces. Accumulation of moisture in the vest padding also leads to breakdown, which requires regular cleansing under the vest.
Truncal Orthoses—These types of orthoses are utilized in the protection of the spine after trauma, surgery, degenerative disease, or congenital disease. The area in which they are applied and stabilize defines the type of truncal orthoses and includes thoracic, lumbar, and sacral regions. Examples are the cruciform anterior sternal hyperextension orthosis (CASH), a three-point pressure system for controlling spine flexation, or the thoraco-lumbo-sacral orthosis (TLSO) or clam shell. Currently, there is a lack of literature examining truncal orthoses and pressure ulcers. All of these braces have varying degrees of skin surface contact, and any areas in which the brace contacts the skin place the patient at risk for pressure ulcer development. This is in part due to the moisture held in the protective padding. As with any type of orthoses, skin and device cleansing should be completed regularly. Health care providers who are not familiar with these orthoses are often fearful of compromising the patient’s safety when attempting regular cleansing. Patient safety is a factor in removal of a stabilizing orthosis; however, it should not negate regular maintenance and cleansing. The orthotist or manufacturer of the orthoses is a good resource on maintaining patient safety while cleansing the underlying skin. As in the case with the cervicothoracic orthosis, any area where the edge meets the skin without padding increases the risk for skin breakdown. It appears that thin or cachectic patients are at higher risk for pressure ulceration secondary to decreased tissue between the interface of the boney spinal prominences and the orthosis. While the design of these orthoses is intended to inhibit movement, the rigid nature of the brace may contribute to pressure ulcer formation. The forces exerted by the brace move the skin and muscle over the underlying structures, causing shearing forces, and when the padding rubs over the surface of the skin, it causes friction forces. These forces in combination with pressure will lead to pressure ulcer development if uncorrected. The patient should be carefully monitored to identify early signs of skin breakdown, including patient complaints of discomfort or improper fitting. In this case, the orthotist should be notified to reevaluate fit or the need for a different orthosis.
Pelvis—Pelvic orthoses or binders are intended to stabilize the pelvis after fracture. The areas most at risk for breakdown are the greater trochanters, iliac crests, sacrum, as well as areas with trapped moisture. Pelvic orthoses cause shearing forces upon application and have continuous high pressures at interface.9 Jowett and Bowyer concluded that thinner patients were at greater risk for pressure ulcer development. It is recommended to not overtighten the pelvic orthosis, there should be enough room for two fingers to fit between the patient’s body and the orthosis, it should not be kept in place longer than necessary, and frequent skin checks, especially at high-risk areas, are warranted.9
Upper Extremities—Elbow, wrist, and hand orthoses stabilize and protect the joints due to instability, trauma, and surgery. They can aid in increasing range of motion when contractures are present. There are many variations of elbow orthoses from single bands to large orthoses with multiple straps. As with any orthosis, moisture from prolonged skin contact can be detrimental to skin integrity. The straps that firmly secure the orthosis can restrict blood flow, creating linear pattern pressure ulcers.
Areas around the elbow and wrist have only thin layers of skin covering boney prominences. As a result of this thin tissue, hard plastic or metal exposed over these boney prominences is vulnerable for pressure ulceration. Wrist orthoses, when used to place the joint in a neutral position to prevent contractures, can have increased forces and moisture where the palmar surface of the hands and the orthosis interface. The first dorsal web space is also susceptible to excessive pressure from ill-fitting orthoses. Currently, the literature does not thoroughly examine upper extremity orthoses and pressure ulceration. It is unclear how often these devices should be rotated or for what length of time a device should remain in place. Regular skin checks and cleansing are the first steps in prevention.
Lower Extremities—Orthoses for the lower extremities span the entire leg from hips to toes. These orthoses can be used for stabilization after trauma or surgery, instability of the joint, increasing range of motion, correction or accommodation of deformities, neurological diseases, or preventing and treating pressure ulcers. Examples of common knee orthoses include dynamic splinting and functional bracing. Boney prominences around the knee and the anterior portion of the lower extremity are at greater risk for breakdown when exposed to hard plastic or metal from the orthosis. The straps used to secure the device can also cause pressure ulceration.
Ankle and foot orthoses encountered in practice include the walker boot, custom-molded shoes, range of motion devices, and semirigid and rigid ankle orthoses. The skin covering the medial and lateral malleoli is thin and is at greater risk for pressure ulceration. Any orthoses in this vulnerable area increase the likelihood or risk of pressure ulceration, and these areas should be closely monitored for skin breakdown. Specialty shoe orthoses are frequently used for patients with diabetic neuropathy and for foot deformities such as Charcot foot. An irregularity in the foot’s shape adversely alters the normal pressure pattern in the foot. Pressure on the toes, metatarsals, and plantar surface increases callus formation and pressure ulcer risk. A Cochrane review in 2000 reported that foot orthoses may be beneficial in preventing ulcers and treating calluses in diabetic patients; however, more research needs to be conducted.10 More recently, a study found that total contact inserts when combined with metatarsal pads decrease the pressure on the metatarsal heads.11 This may prevent pressure ulcer formation on the plantar foot surface in people with diabetes.
Heel orthoses are typically used to offload the heels while in supine and to decrease the risk of pressure ulcer formation. They are typically used for patients with limited mobility. There is a wide range of products available on the market from soft to rigid. Examples include multipodus boots and heel protectors. Although these orthoses are intended to prevent and treat pressure ulcers, they place the patient at risk for developing pressure ulcers from the device itself. Rigid devices expose the posterior ankle/calf tissues and achilles tendon to higher compressive pressures, thus increasing the risk of pressure ulcer development. In addition, despite the intent of rigid orthoses, they do not always completely offload the heel. However, they can be easily molded for a more custom fit. It is often forgotten that soft heel protectors can also cause pressure ulcers from the strap over the anterior ankle or dorsum of the foot. The devices should not be left on for long periods of time. Frequent removal of the orthosis, skin inspection, and cleansing are necessary.12 The foot can be rested on a pillow to offload the heel while in bed. Using a pillow for bed-bound patients is the most effective way to reduce heel pressure followed by heel protectors.13,14
UNLOADING THE PRESSURE
Even though pressure ulcer scales may not define a patient as being at risk for the development of pressure ulcers, any patient with an orthosis is potentially at increased risk. It is imperative that the entire health care team educate the patient and implement methods to prevent orthosis-related skin breakdown. As with any pressure ulcer, prevention is key. Unloading pressure, keeping a clean dry environment, and frequent skin checks are the best practice for the early identification and prevention of pressure ulcers from orthoses.
Kevin R. Emmons, MSN, RN, CWCN, is a clinical nurse specialist and wound specialist at Good Shepherd Penn Partners, Philadelphia. He is also a doctoral nursing student at Drexel University, Philadelphia. Elena Newland, PT, MS, is a senior staff physical therapist who specializes in acute rehabilitation at Good Shepherd Penn Partners. For more information, contact .
REFERENCES
- Medicare Program: Changes to the Hospital Inpatient Prospective Payment Systems and Fiscal Year 2008 rates. Federal Register. 2007;72(162):47129-48175.
- Pressure Ulcer Definition and Stages. Washington: National Pressure Ulcer Advisory Panel; 2007.
- Stechmiller JK, Cowan L, Whitney JD, et al. Guidelines for the prevention of pressure ulcers. Wound Repair Regen. 2008;16(2):151-168.
- Medicare Payments for Orthotics: Carrier Perspectives. Washington: Office of Inspector General; 2000. OEI-02-99-00121.
- Powers J, Daniels D, McGuire C, Hilbish C. The incidence of skin breakdown associated with use of cervical collars. J Trauma Nurs. 2006;13(4):198-200.
- Ackland HM, Cooper JD, Malham GM, Kossman T. Factors predicting cervical collar-related decubitus ulceration in major trauma patients. Spine. 2007;32(4):423-428.
- Jacobson TM, Tescher AN, Miers AG, Downer L. Improving practice; efforts to reduce occipital pressure ulcers. J Nurs Care Qual. 2008;23(3):283-288.
- Belanger L, Cobb J, Bernardo A, Ang R, Adams S, Handfield S. In search of the “superior” cervical orthosis: Philadelphia cervical orthosis versus Aspen cervical orthosis. SCI Nursing. 2004;21(3):158-160.
- Jowett AJ, Bowyer GW. Pressure characteristics of pelvic binders. Injury. 2007;38:188-121.
- Spencer SA. Pressure relieving interventions for preventing and treating diabetic foot ulcers (review). Cochrane Database of Systematic Reviews. 2000(3):1-21.
- Mueller MJ, Lott DJ, Hastings MK, Commean PK, Smith KE, Pilgram TK. Efficacy and mechanism of orthotic devices to unload metatarsal heads in people with diabetes. Phys Ther. 2006;86(6):833-842.
- Black J. Preventing heel ulcers. Nursing. 2004;34(11):17.
- De Keyser G, Dejaeger E, De Meyst H, Eders GC. Pressure-reducing effects of heel protectors. Advances in Wound Care. 1994;7(4):30-32.
- Tymec AC, Pieper B, Vollman K. A comparison of two pressure relieving devices on the prevention of pressure ulcers. Advances in Wound Care. 1997;10(2):39-44.