US Marine SSgt James Sides will reportedly serve as the first subject to receive an implantable myoelectric sensor (IMES) system in a new US Food and Drug Administration (FDA) study. According to a news release from the Alfred Mann Foundation (AMF), the experimental system may have implications as a minimally invasive, intuitive, multi-channel control system for prosthetics intended for long-term use.
The release notes that the IMES System is currently being studied under the Investigational Device Exemption (IDE) regulations of the FDA. AMF notes that in its ongoing trial with injured veterans at the Walter Reed National Medical Military Center, it anticipates subjects operating three prosthetic movements simultaneously. These movements include the opening and closing of the hand, rotating the wrist, and moving the thumb. The combination of these movements would allow for several grasps key for performing daily tasks, the release says.
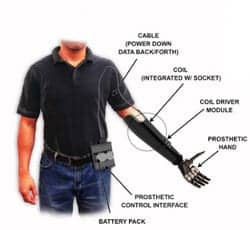
AMF states that the investigational IMES System is engineered to take advantage of electro-chemical signals that occur during muscle contraction. The IMES implants are small capsules measuring 16 millimeters long and 2 millimeters wide. They are placed directly into the residual muscles of an amputated limb and are designed to detect contractions that are then used to facilitate specific movements in the part of the limb that is no longer there. Once the signals are captured, AMF says, they are wirelessly transmitted from the implanted sensor to a decoder box. Ultimately, the system aims to serve as bridge from the brain to the artificial limb, allowing signals to be sent from the brain to the remaining portions of the amputated muscles. This provides control of the prosthesis without the need for brain or muscle reinnervation surgery, the release states.
While the current IMES system is built to provide three distinct movements simultaneously, researchers say future systems will target up to 13 simultaneous degrees of freedom and the ability to blend pre-programmed patterned movements.
“I was thrilled with the idea of collaborating with AMF and leading the IMES project,” says Paul F. Pasquina, MD, retired Army Colonel, principal investigator and chair of the Department of Physical Medicine & Rehabilitation at the Uniformed Services University, former Chief of Orthopaedics & Rehabilitation at Walter Reed National Military Medical Center.
“It holds great promise for the future of amputees…I couldn’t be happier with how quickly our first subject has adapted,” Pasquina adds.
David Hankin, chief executive officer of AMF, calls the work exciting and promising, “and the AMF team of IMES investigators are confident that this technology will result in a new and improved class of prosthetic devices for both upper and lower limb amputees.”
See the system in action here
[Photo Credit: AMF]
[Source: AMF]