An interdisciplinary approach to care and the roles of occupational, physical, and speech therapy.
By Christine DeFiglio, OTR, BCPR; Lindsay Ashmont, DPT; Anthony Lee, MD; and Jessica Dallas, MS, CCC-SLP
According to the American Heart Association, cardiovascular diseases are some of the leading causes of death in the United States.1 Heart failure (HF) has one of the highest mortality rates with 50% of people not surviving more than 5 years after a HF diagnosis.1 The cost of treating heart failure is high, with over 30.7 billion dollars a year spent on treatment and hospitalization.1 Management of heart failure will remain a significant concern for the United States health care system. Currently there are 6.5 million Americans living with heart failure, and 10% have advanced heart failure.1 Heart failure is a growing health care issue and is a leading cause of many cardiovascular surgical interventions, including placement of internal defibrillator, left ventricular assist device (LVAD) placement, and heart transplants.
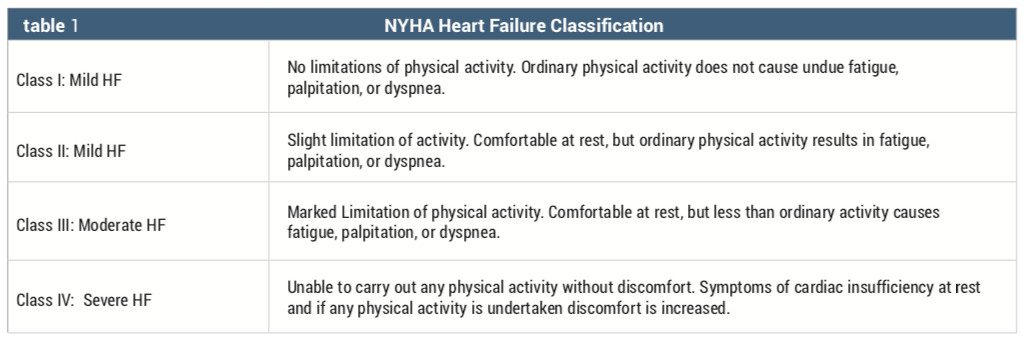
Heart failure can be categorized into four stages in order to determine the best course of therapy. The New York Heart Association (NYHA) has a functional classification system that relates symptoms to everyday activities and the patients’ quality of life (See Table 1).
In the early stages of heart failure, patients are treated pharmacologically. However, with progression of the disease and occasional noncompliance, these patients usually decline, requiring more hospital admissions and progress into the latter stages of heart failure. Some patients in NYHA Class III or IV are candidates for heart transplants. However, waiting for a donor heart can be a long process due to the lack of donor organs. There are other options more readily available to patients with end stage heart failure, which may help prolong a patient’s life overall or until transplantation is possible.
A ventricular assistive device (VAD), either right, left, or both, can be implanted in the end stage heart failure patient for a bridge to transplantation, destination therapy, or recovery. Destination therapy is decided when an LVAD is implanted into an end stage heart failure patient and there is no option for heart transplant.3 Bridge to transplant occurs when a patient is awaiting a heart transplant, and bridge to recovery is when the HF patient has had an LVAD implantation and the LVAD is removed with recovery of heart function without mechanical assistance.3 In 2008, the LVAD was approved by the FDA to be used for both a bridge to transplant and destination therapy.3 There are two types of LVAD devices currently being used: continuous flow left ventricular devices (CF-LVADs) and pulsatile flow left ventricular assist devices (PF-LVADs)(Table 2)6,7,8 Continuous flow devices are based on axial and centrifugal design and are the most popular due to their small size, increased reliability, and higher durability.
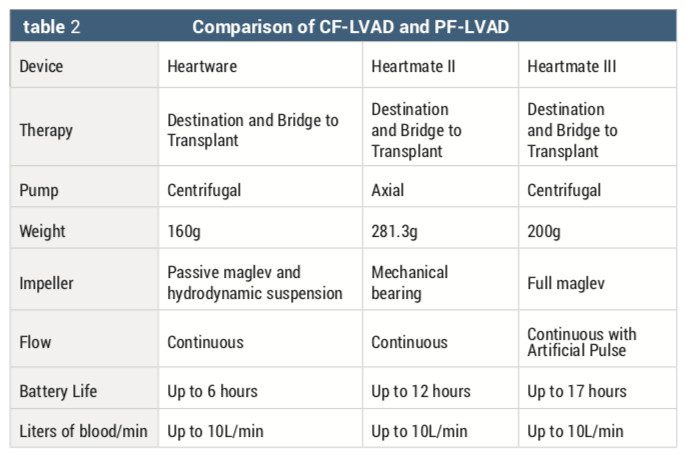
Comparisons between pulsatile and continuous flow LVADs have shown that second generation (continuous flow) LVADs have improved outcomes in terms of survival and decreased incidence of adverse events, such as infection and device malfunction.5
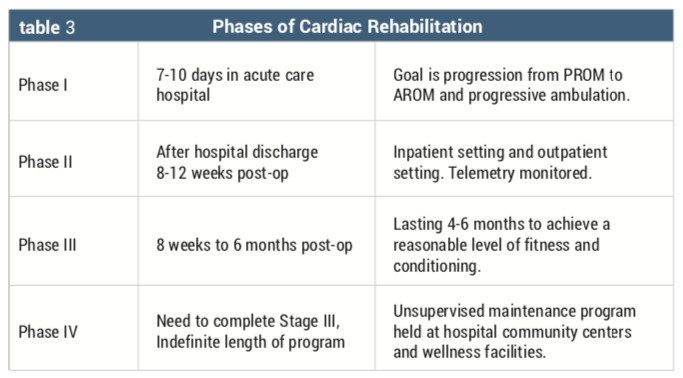
Cardiac rehabilitation is an important component of the recovery of any patient post-cardiac surgery or cardiac event, but it is especially significant for patients post-LVAD placement. Cardiac rehabilitation has multiple phases for a graded recovery process. There are four phases of cardiac rehabilitation (Table 3). Acute inpatient rehabilitation of the LVAD patient falls into phases II and III of the cardiac rehabilitation phases.
Individuals post-LVAD placement that are unable to return directly home from the acute care hospital may require additional recovery time in an inpatient rehabilitation setting. The inpatient rehabilitation setting allows patients to improve their level of function, regain strength, and return to independence in all their activities of daily living. Patients post-LVAD placement exhibit multiple functional deficits including decreased UE and LE strength, decreased fine motor coordination, poor endurance, inability to perform ADLs, and difficulty with ambulation.
There are limited studies that have examined the effectiveness of acute inpatient rehabilitation for patients who have undergone LVAD placement. English et al examined admission and discharge functional independence measures (FIM) of 20 people post-LVAD, and this study showed that most patients had improved function and ability to perform ADLs when discharged home.8 Forrest et al also performed a retrospective review of patients admitted to an inpatient rehabilitation facility after LVAD placement, finding that inpatient rehabilitation is effective for FIM change and community discharge.9 Chu et al completed a retrospective study that examined a 3-year span of patients post-LVAD implantation that were admitted to an inpatient rehabilitation facility (IRF).1,3 Chu concluded that these patients demonstrated functional gains in the IRF; however, they saw a high incidence of acute care transfers due to complications.13 Nguyen and Stein also confirmed the importance of inpatient rehabilitation with post-LVAD patients; their retrospective study showed that these patients achieved clinically meaningful functional gains which were reflected in significant FIM score increases.1,2
Early Activity and Interdisciplinary Care
Research has shown that early active rehabilitation in patients with implanted LVAD improves condition, favorably impacts the clinical course while they await heart transplant, and also improves post-transplant recovery.3 At an IRF, patients post-LVAD placement are provided with the opportunity to improve their daily function by participating in an interdisciplinary program. The patient is provided with medical services; nursing care; psychology services; and physical, occupational, and speech therapy. Communication between disciplines in the inpatient setting as well as the LVAD team from the acute care hospital is crucial with the recovery of the LVAD patient, especially with changes in parameters, fluctuations in vital signs, and other medical management issues.
Individuals admitted post-LVAD and their family members are highly educated in the care of the device and in the drive line to independently perform their connections to and from the main unit and change the batteries. If the person is cognitively impaired or unable to manage their device, the family member or caregiver is responsible for the care of the LVAD device.
Each discipline plays a pivotal role in the rehabilitation of the individual post LVAD placement. Within an IRF the team works closely together to optimize the patient’s rehab stay to improve independence. The goal in the acute rehab setting is to ultimately discharge the patient home, independent in all functional tasks. The physiatrist leads the rehabilitation team in setting goals, medical care, and discharge planning.
The goal of the medical care of patients with LVAD in the rehabilitation setting varies somewhat from the acute care hospital. In the acute care hospital, after a new LVAD is implanted, typical areas of focus include postoperative recovery and optimization of the LVAD’s speed parameter. Although elements of the above goals are incorporated into the continued medical care of LVAD patients while admitted for inpatient rehabilitation, there is also the need to ensure the patient is overall cardiovascularly and medically stable enough to participate and progress in his or her therapy program. Specific examples of where medical interventions may be necessary for LVAD patients in the inpatient rehabilitation setting include sustained alterations in LVAD parameters from baseline, blood pressure management, infectious workup, and anticoagulation management.
Four Parameters for LVAD
A basic knowledge of the parameters associated with an LVAD is crucial for any physician caring for these patients. Coordination of care between medical, nursing, and therapy staff is vital as changes in the parameters can occur at any time and may or may not be associated with symptoms. There are four measurable parameters tracked by the LVAD, which typically can be retrieved and read directly from the device itself. These parameters are the speed, flow, pulsatility index, and power.
Speed is a measure of how quickly the propulsion mechanism in the device is circulating the blood. The speed parameter is initially set at the time of LVAD implantation and optimized shortly thereafter in the postoperative period. The device speed is usually optimized based on echocardiogram findings.10 As such, adjustment to the speed parameter is limited in the rehabilitation setting because the equipment necessary to make the adjustment is typically unavailable.
The flow is a measure of the volume of blood circulated over time. Although there is small minute-by-minute variability in the flow value, typically patients have a baseline range. Sustained changes in the flow from the usual baseline may signify issues with the patient’s circulating volume.10 Commonly, this is a decrease in flow from the baseline range, which signifies a decreased circulating volume. This decreased circulating volume may be a consequence of diuretic medications typically used by heart failure patients, or related to other concurrent medical issues such as dehydration from poor oral intake or diarrhea. Medical interventions that can be instituted in the rehabilitation setting for this include adjustment to diuretic medications, judicious administration of intravenous fluid, and/or addressing concurrent medical issues contributing to the dehydration.
The pulsatility index is a calculated parameter, which includes the flow measurement in the equation. As such, changes in the flow can result in corresponding changes in the pulsatility index. Similar to other parameters, there is minute-by-minute variability in the pulsatility index value, but again, patients typically have a baseline range. Decreases in circulating volume would decrease left ventricular filling and pressure, which in turn could result in sustained decreases in the pulsatility index value.10 Common causes of decreased circulating volume in the rehabilitation setting and their associated treatments have been described above.
The power is a measure of how much energy is being used to keep the LVAD running. Changes in other LVAD parameters can cause changes in the power value. For example, if the speed is increased to optimize echocardiogram findings, then the LVAD will require more power to maintain the increased speed. However, sustained increases in the power without changes in other parameters may signify device malfunction, with greatest concern for thrombus formation within the device.10 Medical intervention is paramount in this event as adjustments to anticoagulation management may be necessary, as well as communication to the referring LVAD team.
Blood Pressure Management
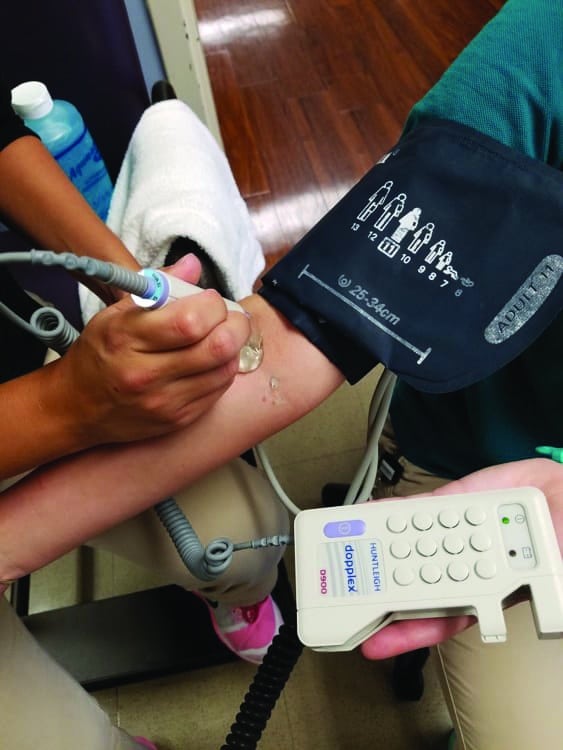
Another area of medical care important in the rehabilitation of LVAD patients is management of their blood pressure. Classically, it is believed that patients with LVADs have continuous flow of blood related to the activity of the LVAD itself. As such, they do not have a true systolic or diastolic blood pressure, and their mean arterial pressure (MAP) is measured with a doppler device.10 As mentioned above, an LVAD is dependent on adequate circulating blood volume for proper function. However, avoidance of sustained elevated blood pressures is similarly important. Related to the continuous flow design of the LVAD, the degree of cardiac support is sensitive to overall vascular resistance.10 An increased after load would make it harder for the LVAD to circulate blood. Oftentimes, patients with LVADs are on medications for management of their underlying heart failure that can concurrently address hypertension. However, if sustained hypertension persists despite use of heart failure medications, then the addition of other medications specifically to target hypertension may be warranted. A MAP goal of 70 mmHg to 80 mmHg, and avoidance of pressures exceeding 90 mmHg, has been reported in the literature for LVAD patients.10 However, in practice, similar to baseline LVAD parameters, each patient appears to have their own unique baseline blood pressure range.
The design and physical components of an LVAD require parts both within the patient’s body and external to the body. Connecting the two is the percutaneous lead. Any time there is a physical connection from outside the body to inside the body, there is an associated risk of infection. Two issues critical to reducing the risk of infection are use of sterile technique when performing dressing changes at the percutaneous lead exit site and adequate immobilization of the percutaneous lead to avoid unnecessary movement.10 Patient and caregiver education on these topics begin with the referring LVAD team during the postoperative period in acute care, but should be reinforced with patient and caregiver in the rehabilitation setting as well.
Furthermore, surveillance for signs and symptoms of infection is also warranted. This includes routine temperature checks to screen for fever, inspection of the percutaneous lead exit site for signs of infection such as erythema or discharge, and periodic laboratory studies to screen for signs of infection such as leukocytosis. If a patient post-LVAD placement demonstrates symptoms of an infection, then a thorough medical work-up is needed to rule out other possible sources of infection common in the rehabilitation setting, such as urinary tract infections and respiratory infections. If other sources of infection have been eliminated and signs of infection persist, then in conjunction with the referring LVAD team, more focus should be placed on evaluation of the LVAD as the potential source of infection.
As an LVAD represents an artificial device in intimate contact with a patient’s cardiovascular system and circulating blood, there is unfortunately an inherent risk for thrombus formation and embolic stroke. Accordingly, all patients with an LVAD are on anticoagulation medication. Oral warfarin is the anticoagulation of choice.10 However, the consensus for the target INR range for the degree of anticoagulation appears to be evolving.10 In practice, the goal INR appears to vary based on the preference of the referring LVAD team. Regardless of the reason why a patient is on warfarin, physicians can appreciate how difficult it can be to maintain a patient steady within a narrow therapeutic range. The INR must neither drop too low, raising the risk for thrombus and stroke, nor elevate too high, raising the risk of bleeding events. This, too, is the case with LVAD patients. As such, close monitoring of laboratory studies and thoughtful dosing of warfarin remains a critical medical intervention in the care of LVAD patients in the rehabilitation setting.
Interdisciplinary Roles of OT, PT, SLP
The rehabilitation nurse has an important role in the care of the patient status post LVAD placement. The rehabilitation nurse is responsible for monitoring vital signs, monitoring the LVAD settings (multiple times throughout the day), monitoring daily weights, and performing dressing changes. The rehabilitation nurse also provides education to the patient and caregiver on dressing changes, signs of infection, the importance of monitoring daily weight, and incision and skin care. Additional education topics include medication management, managing risk factors, smoking cessation, and pain management. The nurse and registered dietician both educate the patient and family on the importance of a heart-healthy diet and diet modifications. Nursing also provides patients discharge information for a smooth transition of care to home.
Besides the physiatrist and rehabilitation nurse, the other key players in the rehabilitation of the patient post LVAD placement is the therapy team. Occupational, physical, and speech therapy each have their own role but work together to maximize the patient’s independence in all functional areas.
Occupational Therapy
Occupational therapists play a vital role in the interdisciplinary acute rehabilitation team treating the patient post LVAD placement. LVAD implantation is a life-changing event that affects the patient’s occupations and roles in life. The occupational therapist addresses all functional components of recovery including strengthening; fine motor dexterity; activities of daily living (ADL’s); instrumental activities of daily living (IADL’s); and leisure, work, and psychosocial aspects.
Upon evaluation, the occupational therapist (OT) performs a complete analysis of occupational performance, taking a complete history of the patient’s current occupations, values, interests, and life roles.11 Prior to initiating the functional components of the evaluation, the OT needs to obtain vital signs (MAP, HR, and O2Sat) via a Doppler and auscultate the LVAD device. A humming sound should be present and documented in the evaluation summary.
The initial evaluation includes a look at the patient’s current ADL and transfer status, upper extremity evaluation (ROM, MMT, sensation, fine motor movements, and grip). Another important component is to evaluate the patient’s ability to manage their LVAD device, including changing the batteries and managing the connectors to the portable unit and main unit (based on the type of LVAD the patient has). All LVAD patients and/or family members have been trained in acute care and should come to inpatient rehab competent in the management of the device. Cognitive assessments should also be done in order to have a baseline of cognition. Common assessments used include the Montreal Cognitive Assessment (MOCA)29 and Hopkins medication management. Vision may also be evaluated if the patient has had a neurological event or a comorbidity of visual deficits.
Goals should be set with the patient to include the patient’s priorities and desired targeted outcomes related to occupational performance, prevention, participation, role competence, health and wellness, quality of life, well-being, and occupational justice.11
The occupational therapist can utilize a number of outcomes measures to assess patient progress through their therapy stay. The Functional Independence Measure (FIM) is utilized to assess a patient’s functional status throughout a rehabilitation stay.22 The Borg Scale of perceived exertion14 is utilized to assess the patient’s feeling of exertion during an activity, which is important with patients post-LVAD where changes in vital signs are not a reliable indicator of activity level. The 9 Hole Peg Test16 and grip strength tests using a dynamometer are also important outcomes to assess the patient’s hand function, which can be decreased in most postsurgical LVAD patients with prolonged hospitalizations.
Numerous treatment interventions are utilized with the LVAD population. The occupational therapist should focus on goal-directed activities including ADL’s, transfers, IADL tasks, UE ROM, UE ROM/strengthening, fine motor coordination, and cognitive tasks. It is important to incorporate the patient’s specific precautions into all functional tasks, which might include sternal and cardiac precautions.
Education is a key component of the patient’s recovery process after LVAD implantation. Cardiac education is also an important role of the occupational therapist with this unique population. This education includes identifying risk factors, establishing lifestyle changes, managing the LVAD device, managing medication, meal planning, home safety, and energy conservation. Education topics that should be included in the OT treatment plan include self-monitoring of vitals, pursed lip breathing, heart-healthy eating, medication management, and use of adaptive equipment or durable medical equipment.
Physical Therapy
The physical therapist (PT) plays a large role in the overall strengthening of the patient and progression of their functional independence. Patients are evaluated within 1 day of their admission. Evaluations consist of obtaining a thorough social history and identifying patient goals; basic testing and screening; assessing functional mobility while monitoring vitals; and orientation to acute rehabilitation and the therapy program. Performance of one or more evidence-based functional outcome measures—the 30-second chair rise test (30CRT)17, the 2-minute walk test (2MWT)18,19,20, the Timed Up and Go (TUG)21,22 the 6-minute walk test (6MWT)33, the Berg Balance Scale (BBS)34; education regarding use of the Borg Scale of Perceived Exertion14; and education on cardiac and sternal precautions. Following completion of evaluation, physical therapists establish goals while considering the patient as a whole. Generally, as with all patients, goals are set with emphasis on maximizing functional mobility and level of independence, while keeping in mind new precautions, the patient’s prior level of function, occupation, and hobbies or interests.
At the beginning and end of every session therapists should auscultate the LVAD device and check the drive line for other wiring for any twisting or crimping to ensure safe positioning and integrity of the device itself. Vitals are obtained taking a patient’s oxygen saturation via pulse oximeter and MAP with use of a doppler and manual cuff. The brachial artery is preferred over radial artery if both are available to assess. Ideal MAP ranges (mmHg) differ from patient to patient. It is recommended that the therapist be aware of the recommended range, as determined by the VAD team at the acute care hospital, so that they may safely exercise within that range or appropriately notify the doctor if outside. Due to the medications that the patient may be on, as well as the functionality of the LVAD device itself, heart rate is not a reliable measure of patient’s exertion. Instead, physical and occupational therapists utilize the Borg14 and/or the Dyspnea Scale34 to assess the patient’s rating of perceived exertion and level of breathlessness. The patient’s physiatrist may also identify a target Borg range to help guide treatment. Oxygen saturation, Borg score14, and patient report of symptoms are monitored with activity and at rest throughout session.
Daily treatment includes a variety of skilled interventions to address deficits and improve upon functional limitations. Progressive therapeutic exercises are completed for strengthening. Patients, especially those classified as bridge to transplant, are often on steroids and benefit greatly from these exercises. It is best practice to obtain approval from the referring VAD team for use of weights for light resistance to optimize results. In addition to therapeutic exercise, a PT should focus skilled interventions on static and dynamic standing balance activities, cardiovascular endurance training, and functional activities (transfers, ambulation, and stair negotiation). If available and necessary, caregivers are identified early on and training is incorporated throughout the patient’s stay.
At time of discharge, PTs assess therapy goal achievement. In addition, PTs re-assess the patient’s functional capacity by performing previously chosen functional outcome measures: 30CRT17, 2MWT18,19,20, 6MWT33, BERG34, and TUG21,22. PTs educate the patients on the clinical significance of their current scores as well as demonstrate change by comparison of their initial scores obtained at evaluation. Finally, precautions, home exercise program, and discharge recommendations are reviewed while necessary durable medical equipment is issued.
Speech-Language Pathology
Speech-language pathology also plays a role in the management of dysphagia and cognitive deficits with the post-LVAD placement patient. It is common for patients with heart failure to exhibit cognitive impairment following cardiac surgery. This is attributable to a variety of factors, including poor cerebral perfusion related to low cardiac output, low systolic blood pressure, and impaired cerebral neurohormonal autoregulation.25 Prevalence of cognitive deficits varies widely, with reports of 25-75% of patients experiencing limitations.25 In patients who have undergone LVAD implantation, cognitive decline has been documented in greater than one of four patients in the year following implantation, with older age and destination therapy being associated with a greater likelihood of cognitive decline.26
A study by Lander et al identified a tendency toward older age and ischemic etiology of cardiomyopathy in the group discharged to a facility.27 It can be reasonably expected that patients with LVADs who will undergo inpatient rehabilitation prior to return home will be older and thus more likely to experience cognitive decline than their younger counterparts.26
Given these demographics, it is reasonable to expect that a significant subset of patients admitted to inpatient rehabilitation post-LVAD implantation will exhibit measurable cognitive limitations. As such, routine cognitive evaluation on admission to inpatient rehabilitation is warranted in this population in order to ensure provision of holistic treatment with the goal of maximizing functional independence at discharge. Speech-language pathologists bring a unique perspective to the evaluation of cognitive status, in that a combination of standardized and non-standardized observations may be considered in the evaluation and treatment of individuals with LVADs.
Common cognitive screens which may be used to evaluate patients with LVADs may include the Mini-Mental State Examination (MMSE)25, the Mini-Cog36, and the Montreal Cognitive Assessment (MoCA).29 While all three assessments are brief (which is desirable in postoperative patients who may still be suffering from the effects of fatigue on endurance), only the MoCA is sensitive to mild cognitive impairment29 and incorporates assessment of executive functioning in addition to memory. Clinically, we see limitations in executive function frequently in patients who have recently undergone LVAD implantation; thus, in our experience, the MoCA is often the most appropriate cognitive screen for this population.
In addition to a structured cognitive screen, it is important to evaluate the patient’s capacity to accurately complete instrumental daily living activities, including management of the LVAD and of medications at discharge. Often, a patient’s family members or other caregivers receive extensive training on LVAD management while the patient is in the acute care setting. For patients who are admitted to inpatient rehabilitation, their acute care stay often is not the ideal time for new learning of these complex concepts due to the probability of experiencing medical complications (as described above). On the other hand, patients with LVADs often cite personal discharge goals of returning to an independent level of function, which will include independent management of their device and medications. Thus, we routinely assess a patient’s knowledge of processes for managing his LVAD during an initial cognitive assessment.
Should limitations in executive functioning or memory be identified during the initial speech-language pathology evaluation, the option for continued speech therapy services to address cognition should be made available to the patient. Regardless of the level of cognitive impairment, treatment tasks should remain functional and incorporate strategies and techniques which can be carried over from the rehab setting to the home environment. Several examples of treatment tasks include completion of power changes (wall power versus battery); daily logging of LVAD parameters in a chart, which can be continued upon discharge home; medication management tasks; and functional problem-solving as related to managing uncommon events (power outages, low flow alarms, controller malfunction, changes in medical status). These an focus on basic strategies, including knowing who to call for help (911, LVAD coordinators, or outpatient cardiologist) and under what circumstances.
Finally, like other patients who have undergone cardiac surgeries, patients with LVADs may present with clinically significant dysphagia as a result of critical illness and prolonged hospitalization.30-32 The speech-language pathologist will evaluate the patient’s ability to tolerate different levels of food and liquid consistencies and textures. A video fluoroscopic swallow study can be performed along with a physician to evaluate progress in a patient swallowing deficit and can determine upgrades in food and treatment to determine or manage strategies to minimize the risk of aspiration and increase swallow efficiency.
Additional Disciplines
Other team members, including case managers, pharmacists, registered dieticians, and psychologists, play important roles in the recovery of the post-LVAD placement. The case manager, along with the interdisciplinary team, patient, and patient’s family, work together to plan the most appropriate discharge for the patient. The case manager helps the patient and family transition from the inpatient acute rehabilitation setting to home. He or she assists the patient with a referral to an appropriate home care agency that has been trained and capable of handling any issues with the LVAD upon discharge, or to outpatient cardiac rehabilitation, if appropriate. The patient is educated on the importance of following up with their LVAD team and also educated on the role of local agencies such as the police, EMS, and utility companies in their transition with an LVAD back home. The case manager also assists the patient with insurance needs, such as authorization for dressing supplies and other medical equipment that may be needed with the transition home. Pharmacists assist the patient in understanding the medication that they are currently prescribed and work along with the nurse, physiatrist, and LVAD coordinator for medication changes. The registered dietician educates the patient on heart-healthy and low-sodium diets while they are an inpatient and teaches them how to follow the diets when they are in their home environment. Psychologists play a large role in the recovery of the patient post-LVAD placement. The psychologist provides emotional support for the patient, focusing on the change in their quality of life, the recovery process, and rehabilitation goals. The psychologist works closely with the therapy team to monitor patient progress, therapy participation, and signs of depression and anxiety; and can provide resources for support once discharged.
The interdisciplinary team is important in the patient’s acute rehabilitation post-LVAD placement. Each discipline plays a significant role in the functional recovery of the patient and have one common goal: to improve functional performance and discharge into the community. Previous studies by Nygen, Stein12, and Chu13 have demonstrated that patients post-LVAD implantation would benefit from an inpatient rehabilitation stay. The interdisciplinary approach is a vital component to a successful inpatient rehabilitation program. Acute inpatient rehabilitation is a crucial step in the rehabilitation of patients post-LVAD, providing the education and physical conditioning necessary for a successful recovery. RM
Christine DeFiglio, OTR, BCPR (AOTA Board Certified in Physical Rehabilitation), is a clinical specialist at Kessler Institute for Rehabilitation in Saddle Brook, NJ, and serves as clinical co-lead for the Cardiac Recovery Program at Kessler Institute for Rehabilitation.
Anthony Lee, MD, is a Clinical Assistant Professor in the Department of Physical Medicine and Rehabilitation at Rutgers – New Jersey Medical School, a Clinical Chief and the Director of Cardiac Services at the Kessler Institute for Rehabilitation, and serves as the medical lead for the Cardiac Recovery Program at the Kessler Institute.
Lindsay Ashmont, DPT, is a Clinical Specialist at Baylor Scott and White Institute for Rehabilitation in Dallas and the clinical lead for the Cardiac Recovery program.
Jessica Dallas, MS, CCC-SLP, is a clinical specialist at Baylor Scott and White Institute for Rehabilitation in Dallas and is the clinical lead for the Left Ventricular Device Committee. For more information, contact [email protected].
References
- American Heart Association News. 2017. Heart Failure Projected to increase dramatically according to new statistics. AHA website, www.heart.org. Jan 25, 2017.
- Raphael, C. et al. Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 2007 Apr; 93(4):476-82.
- Atsuko U, Yasuko T. Cardiac Rehabilitation and Artificial Heart Devices. Journal of Artificial Organs. 2009;12:90-97.
- Compostella, L. et al. A Practical Review for Cardiac Rehabilitation Professions of Continuous –Flow Left Ventricular Assist Devices. Journal of Cardiopulmonary Rehabilitation. 2015;35:301-311.
- Nadziakiewicz, P et al. Comparison of Mechanical Circulatory Support by the Use of Pulsatile Left Ventricular Assist Devices Polvad MEV and Continuous Flow Heart Ware and Heart Mate II in a Single-Center Experience. Transplant Proceedings. 2016;48:1770-1774
- Medtronic.com
- Heartmate II LVAD: The new Era Begins. 2018 Throatec.com
- English, ML, Speed J. Effectiveness of Acute Inpatient Rehabilitation after Left Ventricular Assist Device Placement. AmJPhyMedRehab. 2013;92:621-626.
- Forrest, George et al. Left Ventricular Assist Device: Care on Inpatient Rehabilitation Facility. Rehab Nursing. 2014;40:378-383.
- Slaughter, M., et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. Journal of Heart and Lung Transplantation. 2010;29:S1-S39.
- American Occupational Therapy Association. (2014). Occupational therapy practice framework: Domain and process (3rd Ed.).American Journal of Occupational Therapy, 68(Suppl.1), S1–S48.
- Nguyen E, Stein J. Functional Outcomes of adults with left ventricular assist devices receiving inpatient rehabilitation. The Journal of Injury, Functional and Rehabilitation. 5, 99-103.
- Chu S., McCormick, A. et al. Outcomes of Acute Inpatient Rehabilitation of Patients with Left Ventricular Assist Devices. American Academy of Physical Medicine and Rehabilitation. Vol 6. 1008-1012. November 2014.
- Borg, Gunnar, Psychophysical bases of perceived exertion. Medicine and Science in sports and Exercise. 14(5):337-381, May 1982.
- De jonge, Nicholas MD, et al. Exercise performance in patients with end stage heart failure after implantation of a left ventricular assist device and after heart transplantation. Journal of the American College of Cardiology. Vol. 37, no7, 2001
- Mathiowetz V, Weber K, Kashman N, Volland G. Adult Norms for the Nine Hole Peg Test of Finger Dexterity. The Occupational Therapy Journal of Research. 1985;5:2433
- Rikli, R, Jones C, Functional fitness normative scores for community-residing older adults, ages 60-94. J Aging Phys Activity. 1999:7(2):162-81.
- Butland RJ, Pang J, Gross ER, Woodcock AA, Geddes DM. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J (Clin Res Ed). 1982 May 29; 284(6329):1607-8.
- McGavin CR, Gupta SP, McHardy GJ. Twelve-minute walking test for assessing disability in chronic bronchitis. Br Med J. 1976; 3; 1(6013):822-3.
- Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001; 82(1):9-13.
- Podsiadlo D, Richardson S. The Time “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. Journal of the American Geriatrics Society 1991; 39(2): 142148
- Shumway Cook, A, Brauer S, Woollacott M. Predicting the Probability for Falls in Community Dwelling , Older Adults Using the Timed Up & Go Test. Physical Therapy 2000 Vol 80(9): 896903.
- Mar 2017 Functional Independence Measurement (FIM) User Manual, Version 1.0
- Trombly, Catherine. Cardiopulmonary Rehabilitation. Chapter 7, Occupational Therapy for Physical Dysfunction, 3rd Edition, 1989
- Bhat G, Yost G, Mahoney E. Cognitive function and left ventricular assist device implantation. Journal of Heart and Lung Transplantation, 2015; 34:1398-1405.
- Fendler TJ, Spertus JA, Gosch KL, et al. Incidence and predictors of cognitive decline in patients with left ventricular assist devices. Circ Cardiovasc Qual Outcomes, 2015; 8: 285-291.
- Lander BS, Patel K, Blackstone EH, Nordseth T, Starling RC, Gorodeski EZ. Post-acute care trajectories in the first year following hospital discharge after left ventricular assist device implantation. Journal of the American Medical Director’s Association, 2016; 17: 908-912.
- Pandharipande PP, Girard TD, Jackson JC, Moranid A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, and the BRAIN-ICU Study Investigators. Long-term cognitive impairment after critical illness. N Engl J Med, 2013; 369(14): 1306-1316.
- Mikkelsen ME, Netzer G, Iwashyna T. Post-intensive care syndrome (PICS). https://www.uptodate.com/contents/post-intensive-care-syndrome, February 10, 2020.
- Grimm J C, Magruder JT, Ohkuma R, Dungan SP, Hayes A, Vose AK, Orlando M, Sussman MS, Cameron DE, Whitman GJR. A novel risk score to predict dysphagia after cardiac surgery procedures. The Annals of Thoracic Surgery, 2015; 100: 568-574.
- Daly E, Miles A, Scott S, and Gillham M. Finding the red flags: swallowing difficulties after cardiac surgery in patients with prolonged intubation. Journal of Critical Care, 2016; 31: 119-124.
- Gee E, Lancaster E, Meltzer J, Mendelsohn AH, Benharash P. A targeted swallow screen for the detection of postoperative dysphagia. The American Surgeon, 2015; 81(10): 979-982.
- Hasin T., Topilsky Y., Kremers W.K., et al. Usefulness of the six-minute walk test after continuous axial flow left ventricular device implantation to predict survival. Am J Cardiol. 2012; 110: 1322-1328
- Manali ED, Lyberopoulos P, Triantafillidou C, et al. MRC chronic Dyspnea Scale: Relationships with cardiopulmonary exercise testing and 6-minute walk test in idiopathic pulmonary fibrosis patients: a prospective study. BMC Pulm Med. 2010; 10:32. doi:10.1186/1471-2466-10-32
- Folstein, MF; Folstein, SE; McHugh, PR (1975). “”Mini-mental status”. A practical method for grading the cognitive state of patients for the clinician”. Journal of Psychiatric Research. 12 (3): 189–98
- Patel, Apurva, R. Parikh, E. Howell, E. Hsich, S. Landers, E Gorodeski (2015) Mini-Cog Performance Novel Marker of Post Discharge Risk Among Patients Hospitalized for Heart Failure. Heart Failure. 8:8-16.





