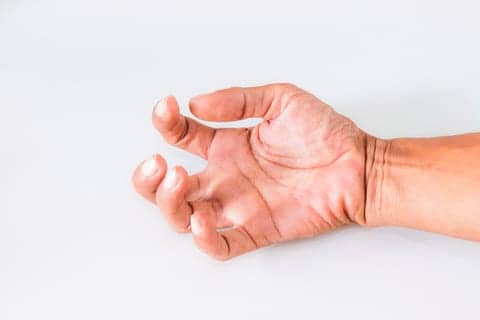
A new type of relief from pain, stiffness, spasms, and in the upper arm that affect millions of adults affected by spasticity has just been approved by the US Food and Drug Administration (FDA). The agency’s approval of Dysport (abobotulinumtoxinA) opens the door to an injection for the treatment of upper limb spasticity (ULS) in adult patients to decrease the severity of increased muscle tone in elbow flexors, wrist flexors, and finger flexors, according to a media release from the drug’s maker Basking Ridge, NJ-based Ipsen Biopharmaceuticals.
The Ipsen Biopharmaceuticals media release outlines what the company identifies as key benefits associated with Dysport. Among those benefits, the company reports Dysport is the first therapy in the past 5 years approved by the FDA for the treatment of adults with ULS. Furthermore, the company states, the Dysport Phase III trial was the first registration study to evaluate ULS treatment in adult patients with both stroke and traumatic brain injury.
Another benefit reported by the company is statistically significant improvement in muscle tone measured by the Modified Ashworth Scale (MAS) and a significantly higher physician-rated clinical benefit measured by the Physician Global Assessment (PGA) versus placebo at Week 4 (p ? 0.05).
According to the media release, clinical improvement may be expected 1 week after administration of Dysport. The company also points out that a majority of patients were retreated between 12 and 16 weeks; however, some patients had duration of response as long as 20 weeks.
The most frequently reported adverse reactions (?2%) are: urinary tract infection, nasopharyngitis, muscular weakness, musculoskeletal pain, dizziness, fall, and depression, the media release states.
All botulinum toxin products, including Dysport, are said by Ipsen Biopoharmaceuticals to have a Boxed Warning which states that the effects of the botulinum toxin may spread from the area of injection to other areas of the body, causing symptoms similar to those of botulism. Those symptoms include swallowing and breathing difficulties that can be life-threatening.
[Source: Ipsen Biopharmaceuticals Inc]