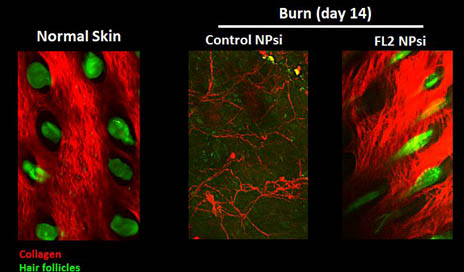
Imaging of burns indicates that those treated with the FL2 inhibitor nanotechnology experienced collagen deposition and hair follicle formation. (2-photo confocal microscopy) Credit: Vera DesMarais/Albert Einstein College of Medicine
The next leap forward in treatment of chronic wounds may spring from a genetic tweak that appears capable of shrinking healing time by half. This accelerated healing, under a study led by David J. Sharp, PhD, at Albert Einstein College of Medicine, New York, is triggered by a nanoparticle that allows skin cells to more quickly reach damaged areas of the dermis.
“We envision that our nanoparticle therapy could be used to speed the healing of all sorts of wounds, including everyday cuts and burns, surgical incisions, and chronic skin ulcers, which are a particular problem in the elderly and people with diabetes,” Sharp notes in a media release from Albert Einstein College of Medicine. Sharp is professor of physiology and biophysics at the college.
Details about the study, which was successfully conducted on an animal model, are published in the Journal of Investigative Dermatology online edition.
Behind the healing is a drug laden with tiny gel capsules—nanoparticles. Within the nanoparticles are molecules of silencing RNA (siRNAs) that shut down the enzyme known as fidgeting-like 2 (FL2). This FL2 enzyme restricts skin cells from moving into a damaged area of tissue to conduct healing. By inhibiting the effects of FL2, skin cells are able to reach wounds quickly and accelerate healing.
According to the college’s media release, Sharp and colleagues discovered that healing among subjects that received the nanoparticle treatment occurred at rates up to twice as fast as control subjects that received no treatment. The nanoparticles with their siRNA cargoes were tested by topically applying them to mice with either skin excisions or burns. In both cases, the wounds closed more than twice as fast as in untreated controls.
“Not only did the cells move into the wounds faster, but they knew what to do when they got there,” Sharp observes. “We saw normal, well-orchestrated regeneration of tissue, including hair follicles and the skin’s supportive collagen network.”
Sharp reportedly plans to soon move testing of the nanoparticle therapy to pigs, which have skin characteristics similar to humans. The paper, published online March 10, is titled “Fidgetin-like 2: a Novel Microtubule-Based Regulator of Wound Healing.” Others who contributed to the study include: Rabab Charafeddine, PhD candidate; Joy Makdisi, MD student; David Schairer, MD; Brian O’Rourke, PhD; Juan D. Diaz-Valencia, PhD; Jason Chouake, MD student; Allison Kutner, MD; Aimee Krausz, MD student; Brandon Adler, MD student; Parimala Nacharaju, PhD; Hongying Liang; Suranjana Mukherjee, PhD candidate; and Joshua Nosanchuk, MD.
[Source: Albert Einstein College of Medicine]



