.png)
Using teriflunomide to treat relapses in MS patients could be a safe, effective and convenient new therapy, says Paul O’Connor, MD, principal investigator of the study and director of the Multiple Sclerosis Clinic at St. Michael’s Hospital in Toronto, Canada.
The results of the Phase 3 trial were recently published in the New England Journal of Medicine.
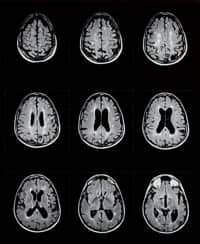
The study encompassed 1,088 MS patients aged 18 years to 55 years who had experienced at last one relapse in the previous year or 2 relapses within the previous 2 years. Researchers conducting the 2-year study treated one-third of the patients with a placebo, one-third with a 7-miligram dose of teriflunomide and one-third with 14 milligrams of teriflunomide.
The researchers’ findings reflected a 31% reduction in relapses in patients taking the drug. The study also reported an increase in the length of time before a patient relapsed and a higher number of patients who remained free of relapses. According to researchers, the progression of the disease was also reduced by 30% among those taking the 14-miligram dose of teriflunomide.
O’Connor says that the patients in the clinical trial tolerated the drug well and displayed no difference in the rate of serious side effects compared to those who took the placebo. Common side effects in the teriflunomide patient population included diarrhea, nausea, and mild hair loss.
Source: St. Michael’s Hospital