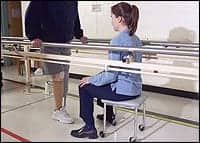
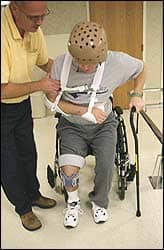
The Emagine trial will examine the effectiveness of a wearable electromagnetic therapy device in accelerating stroke recovery.
Hackensack Meridian JFK Johnson Rehabilitation Institute is researching a wearable electromagnetic therapy device that accelerates healing after a stroke.
JFK Johnson is one of 20 rehabilitation hospitals nationwide enrolling patients in the Emagine Stroke Recovery Trial, which aims to enhance recovery and reduce disability after neurologic damage caused by stroke.
The device targets networks in the brain with a low-intensity, frequency-tuned electromagnetic field therapy. JFK Johnson Rehabilitation Institute is training rehabilitation specialists to guide patients and to train caregivers to use the device. The program consists of 45 hour-long sessions, 5 times each week, with weekly follow-up from a JFK Johnson rehabilitation specialist.
The wearable device can be used in a hospital setting, outpatient clinic, and at home and would augment JFK Johnson’s existing rehabilitation therapies.
“We’re participating in this innovative research and other clinical trials because we’re continually working to maximize the recovery of our patients and advance the science of stroke recovery,” said Sara Cuccurullo, MD, chair, vice president and medical director of JFK Johnson Rehabilitation Institute and principal investigator of the study. “We want all of our patients to reach their highest quality of life.”
The wearable device was given breakthrough status by the FDA after a pilot study showed promise. The study is funded by BrainQ, the technology company that developed the investigational device.
JFK Johnson treats approximately 750 stroke patients each year. Stroke is a leading cause of serious, long-term disability. Every 40 seconds, someone in the United States experiences a stroke, according to the U.S. Centers for Disease Control and Prevention.
“The patients we’re talking to are interested and tell us they want to do whatever they can to recover as fully as possible,” said Maria Belen Montealegre, OTD, OT/L, CSRS, Occupational Therapy Supervisor and Site Coordinator of the clinical trial.
The Emagine trial will enroll 150 randomized subjects nationwide within four to 21 days following a stroke.
“Stroke can be devastating. Improving outcomes for people recovering from stroke will have enormous impact for individuals and their families as well as far-reaching impact for our nation,” said Hayk Petrosyan, PhD, senior research scientist at JFK Johnson Rehabilitation Institute.
Photo 162568223 © Robert Kneschke | Dreamstime.com