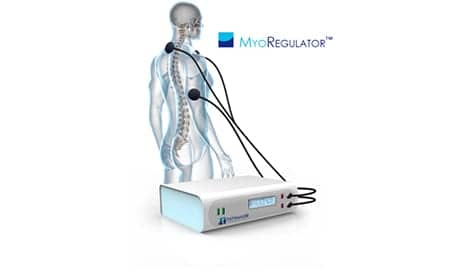
PathMaker Neurosystems Inc announces the receipt of a nearly $5 million cooperative agreement from the National Institutes of Health to advance the development of its MyoRegulator device as a treatment for spasticity secondary to stroke.
The 4-year grant has been awarded through the CREATE Devices program that provides a collaborative partnership with the National Institute of Neurological Disorders and Stroke (NINDS), according to the release.
“We are truly honored and delighted to receive this support from NINDS to advance our breakthrough non-invasive neuromodulation technology that can benefit many patients in desperate need of treatment options,” says Nader Yaghoubi, MD, PhD, president and chief executive officer of PathMaker Neurosystems, in the release.
“This collaborative partnership and funding will support product engineering, multi-center US pivotal trials and regulatory submission for MyoRegulator.”
MyoRegulator is a non-invasive neuromodulation device based on PathMaker’s proprietary DoubleStim technology, which provides simultaneous noninvasive stimulation at spinal and peripheral sites to durably reduce muscle spasticity.
“The CREATE Devices program encourages the pursuit of translational and clinical studies for innovative therapeutic devices to treat neurological disorders,” states Nick Langhals, PhD, program director at NINDS, per the release.
[Source(s): PathMaker Neurosystems Inc, Business Wire]