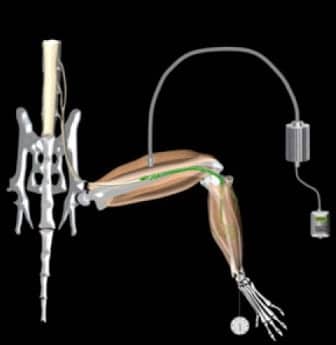
Scientists at University College London (UCL) and King’s College London have reportedly developed a strategy designed to artificially control muscles using light, which may potentially pave the way to restoring function to muscles paralyzed by conditions such as motor neuron disease and spinal cord injury (SCI). The technique, according to a news release from UCL, encompasses the transplantation of specially designed motor neurons created from stem cells, into injured nerve branches.
The release notes that the motor neurons are engineered to respond to pulses of blue light, allowing scientists to fine-tune muscle control by adjusting intensity, duration, and frequency of the light pulses. During the study, the team states that it demonstrated the approach in mice in which the nerves that supply muscles in the hind legs were injured. The results indicate that transplanted stem cell-derived motor neurons grew along the injured nerves to connect successfully with the paralyzed muscles, which could then be controlled by pulses of blue light.
Linda Greensmith, PhD, professor, MRC Centre for Neuromuscular Diseases at UCL’s Institute of Neurology, led the study. Greensmith notes that the new strategy “has significant advantages over existing techniques that use electricity to stimulate nerves, which can be painful and often result in rapid muscle fatigue. Moreover, if the existing motor neurons are lost due to injury or disease, electrical stimulation of nerves is rendered useless as these too are lost.”
The release reports that Ivo Lieberam, PhD, MRC Center for Developmental Neurobiology, King’s College London, generated the light-responsive motor neurons that made the technique possible from cells. Lieberam explains that the scientists tailored the embryonic stem cells in order to allow the motor neurons derived from them to function as part of the muscle pacemaker device.
“First, we equipped the cells with a molecular light sensor. This enables us to control motor neurons with blue light flashes. We then built a survival gene into them, which helps the stem-cell motor neurons to stay alive when they are transplanted inside the injured nerve and allows them to grow to connect to muscle,” Lieberam says in the release.
Greensmith adds that within the next 5 years or so, the scientists hope to undertake the next key steps required to take the approach into human trials, “potentially to develop treatments for patients with motor neuron disease, many of whom eventually lose the ability to breathe, as their diaphragm muscles gradually become paralyzed. We eventually hope to use our method to create a sort of optical pacemaker for the diaphragm to keep these patients breathing.”
Photo Credit: Barney Bryson
Source: University College London