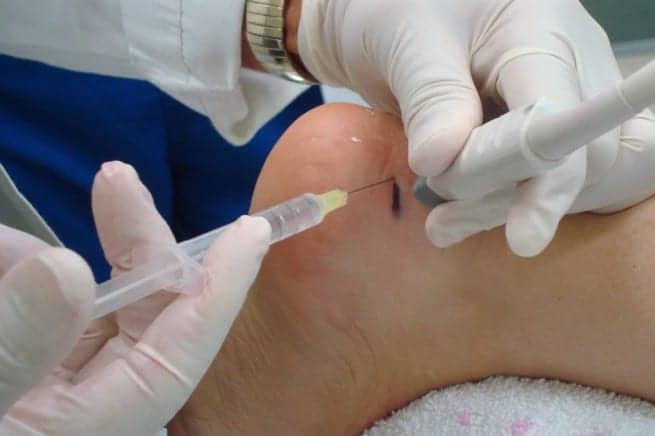
RepliCel Life Sciences Inc announced that it will progress to clinical trials with its injectable treatment to address adverse effects associated with chronic tendinosis. The trial will include 28 patients affected by chronic Achilles tendinosis who have completed physical therapy with no improvement, according to a media release from RepliCel.
“We will be giving [each study subject] a single injection, directly into the Achilles tendon using ultrasound guided imaging, of replicated collagen producing fibroblast cells isolated from the dermal sheath of their own hair follicles,” says David Hall, chief executive officer of RepliCel.
The company states that the injection of fibroblasts aims to promote the healing process by producing proteins that are necessary for restoring the structural integrity of tissue as well as type I collagen fibers which orientate themselves along the direction of the stretch of the tendon.
“The anticipated long-term outcome is the return of normal tendon structure, improved function and zero pain,” Hall adds.
In a media release from RepliCel Dr. Ross Davidson, Chair of RepliCel’s RCT-01 Clinical Advisory Board and retired Clinical Professor, Department of Orthopaedics, UBC, stated that Health Canada’s No Objection Letter for RepliCel’s ReaCT trial within 30 days of the Company’s first clinical trial application supports the technology and represents a vote of confidence for the clinical team in terms of the trial design. “I expect this trial focused on chronic Achilles tendinosis to be the first step in addressing other debilitating tendon injuries in the future,” Davidson says.
More information is available at the company’s website.
[Source: RepliCel Life Sciences Inc]