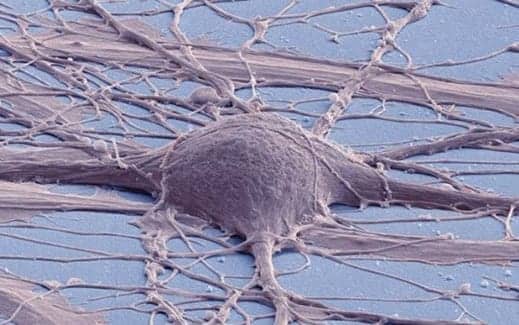
Pictured here is a scanning electron micrograph of cultured human neuron from induced pluripotent stem cell. (Photo courtesy of Mark Ellisman and Thomas Deerinck, National Center for Microscopy and Imaging Research, UC San Diego)
Researchers share their successful creation of spinal cord neural stem cells (NSCs) from human pluripotent stem cells (hPSCs) that differentiate into cells capable of dispersing throughout the spinal cord and can be maintained for long periods of time.
This achievement may constitute an improved, clinically translatable cell source for replacement strategies in spinal cord injuries and disorders, according a media release from University of California – San Diego.
In the study, published recently in Nature Methods, first author and postdoctoral scholar Hiromi Kumamaru, MD, PhD, and senior author Mark Tuszynski, MD, PhD, professor of neuroscience and director of the UC San Diego Translational Neuroscience Institute, and colleagues describe the creation of the cell line.
After grafting cultured hPSC-derived NSCs into injured spinal cords of rats, they noted that the grafts were rich in excitatory neurons, extended large numbers of axons over long distances, innervated their target structures and enabled robust corticospinal regeneration.
“We established a scalable source of human spinal cord NSCs that includes all spinal cord neuronal progenitor cell types,” Kumamaru says, in the release. “In grafts, these cells could be found throughout the spinal cord, dorsal to ventral. They promoted regeneration after spinal cord injury in adult rats, including corticospinal axons, which are extremely important in human voluntary motor function. In rats, they supported functional recovery.”
Tuszynski notes that, although more work needs to be done, these newly generated cells will constitute source cells for advancement to human clinical trials on a time frame of 3 to 5 years. It still needs to be determined that the cells are safe over long time periods in rodent and non-human primate studies, and that their efficacy can be replicated.
He adds that the work presents potential benefits beyond spinal cord injury therapies since the NSCs can be used in modeling and drug screening for disorders that also involve spinal cord dysfunction, such as amyotrophic lateral sclerosis, progressive muscular atrophy, hereditary spastic paraplegia and spinocerebellar ataxia, a group of genetic disorders characterized by progressive discoordination of gait, hands and eye movement, per the release.
[Source(s): University of California – San Diego, Science Daily]