 |
Transdermal and topical delivery of drugs through the skin can be assisted by electrical energy. Iontophoresis has been used with steroids, nonsteroidal anti-inflammatory drugs, local anesthetics, and salicylates to produce analgesia in a variety of clinical settings. Iontophoresis uses a low-amplitude electrical current to drive ionic drug molecules through the skin. The applied electrical current will split water into H+ and OH– in a process known as hydrolysis. A method of buffering the pH changes due to hydrolysis is necessary in all iontophoresis electrodes. The pH control system keeps the native pH of the drug solution relatively constant. This is important as the charge of the drug may be altered with pH shifts. Since it is the charge that propels the drug in an electrical field, the efficiency of delivery is altered by any changes in the polarity of the drug molecule. Iontophoresis is usually very well tolerated by most patients. Contraindications for use include:
- Previous adverse reaction or hypersensitivity to electrical stimulation.
- Sensitivity to the administered medication.
The most common problems encountered with iontophoresis are skin irritation and slim possibilities of burns. Excessive amperage can lead to thermal injury.
PHYSICS RELATIONSHIP TO BURN INJURY
Voltage and current are proportionally related to one another by Ohm’s law represented by the equation voltage (V) = current (I) x resistance (R). The resistance (measured in ohms) is a material property that tends to impede the flow of electrons. Thus, current flow through a material or tissue is proportional to the voltage applied and inversely proportional to the material’s resistance (I = V/R). Although artificially reproduced and not entirely clinically applicable, animal experimentation has provided some insight into the mechanism of injury caused by electrical incidents. During electrical contact, resistance varies continuously with time, initially dropping slowly and then rapidly until arcing occurs at the sites of contact. Current flow increases while the resistance drops and temperature rises at the contact site. As the temperature increases and thermal damage occurs, desiccation and carbonization of the surrounding tissue occur and, therefore, the resistance increases significantly. The tissue injury is caused primarily by thermal damage, and severity is proportional to the magnitude of temperature increase. Temperature changes during application of current occur at the contact points.
Many self-integrated electrodes with a battery on board entered the rehabilitation market over the last 5 years. These electrodes use a low-voltage battery source on board, which reduces the chance of burns. However, these electrodes do not have the ability to control the current, Ohm’s law relationship V = IR. Thus, the drug dosage delivered to the patient could vary significantly depending on the patient’s skin resistance and other variables. Traditional dose controllers with microprocessors on board precisely control the current and dosage. The traditional controllers accommodate for the variable skin resistance by adjusting the voltage to maintain the current at a constant rate. Therefore, the drug dosage delivered to the patient is precise and exact regardless of any variables in skin resistance. It was reported in the literature that the average resistance for human skin is 25,000 ohms/cm2. There are currently several self-integrated electrodes in the pharmaceutical markets that are microprocessor controlled. These sophisticated self-integrated electrodes are inherently costly and, therefore, sensitive to the reimbursement challenges facing the rehabilitation market.
AVOIDING INTERNAL HIGH CURRENT DENSITIES
Whenever the path of electrical current (mA) is constrained or constricted, the current density (mA per cm2) increases. Increases in current density may cause skin irritation or burns. High current densities may occur internal to the electrode if the drug reservoir is not uniformly hydrated. As only the wetted areas of the drug reservoir conduct current and if the hydrated area is not uniform, a high current density results. High current densities also may occur if the adhesive seal of the electrode with the skin does not adhere well and leaks. Again, the drug reservoir may become nonuniformly wet, resulting in high current densities.
AVOIDING EXTERNAL HIGH CURRENT DENSITIES
High current density conditions outside of the electrode generally occur as local short circuits and as a result of inadvertent externally applied pressure over the drug delivery and/or ground electrode(s).
High current densities caused by a short circuit are often the result of (metal) jewelry worn in the vicinity of the electrode. Metal provides a low resistance channel for current to be routed away from the lead, snap, or electrode. If the metal also touches the skin, a high current density pathway is created that may result in a skin burn.
High current densities caused by externally applied pressures such as hot packs, cold packs, elastic bandages, etc, are the result of compressing the conductive element within the drug delivery and/or ground electrode(s). The compression over the electrode causes uneven current distribution, which may cause skin irritation or burns. Never tape, bind, or compress either electrode during an iontophoresis procedure.
PREPARATION OF TREATMENT SITE
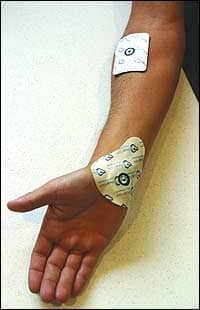 |
| Treatment electrodes should be carefully applied directly on the affected area. |
The treatment site should be carefully prepared. The skin should be cleansed with alcohol. The treatment site should be free of trauma and excessive body hair. Shaving can cause micro abbreviation to the skin so clipping excess hair is recommended. Electrode placement is important and the electrodes should be applied carefully. The treatment electrode should be placed directly over the area to be treated if possible.
The electrodes should be well adhered to the skin. Loose electrodes can contribute to thermal burns and skin irritation. The patient should not extend or flex the area being treated or press or lean against the electrodes during treatment. The electrodes should be secured; however, never bind or tape the electrodes to the treatment area. Electrodes should never be reused.
FDA REGULATIONS
The objective of the Food and Drug Administration (FDA) is to assure that the device is equivalent to a predicate device and is as safe and effective as a predicate device on the market. The FDA clears iontophoresis medical devices via a 510(k), a process in which the manufacturer has to prove the safety and efficacy of their medical device to the predicate medical device by a series of tests and labeling. It restricts these devices to sale by or on the order of a physician. The FDA allows companies to market these devices as intended to be used for the administration of soluble salts into the body for medical purposes and as an alternative to hypodermic injection. The FDA will allow a specific drug to be mentioned if and only if it has been cleared via an NDA (New Drug Application). Currently, there are a few medical iontophoresis devices approved via an NDA for the delivery of lidocaine and fentanyl.
ADVANTAGES OF IONTOPHORESIS
Iontophoresis has the advantages over conventional administration of medication of avoidance of systemic administration of medication, thus no systemic side effects. Studies have demonstrated undetectable systemic blood concentrations. Locally, there is diffuse drug administration without multiple hypodermic punctures or tunneling to increase injection area, which is ideal for drug deposition around inflamed tendons and diffusion into joints.
Recently, newer systems provide the following advantages to improve drug delivery and decrease potential complications:
- Precise pH regulation, allows delivery of the maximal dosage (80 mAmp*min).
- Highly absorbent drug reservoir minimizes the occurrence of high density current pathways.
- Conformable and adherent adhesive layer minimizes leakage of drug being administered.
- Low electrical impedance reduces constrictions of the voltage gradient as current passes through the active electrode.
- Large return electrode area lowers sensation and increases comfort.
- Low profile, drug delivery electrode conforms to complex geometries of the wrist, elbow, knee, finger, and ankle.
- Precise anatomic positioning, saving clinician intervention time.
In summary, iontophoresis can be particularly helpful in providing analgesia in a variety of inflammatory conditions without the complications associated with oral and injectable drug delivery alternatives such as gastrointestinal complaints or adrenal suppression. Recent publicity on the dangerous side effects associated with COX-2 inhibitors and subsequent regulatory action have given physicians fewer traditional treatment alternatives, resulting in increased referrals to physical therapy. Dexamethasone delivered via iontophoresis system remains localized in the targeted tissues, exerting its beneficial effects while avoiding systemic circulation.
When considering the potential side effects associated with oral anti-inflammatory medications and the painful and potentially damaging side effects of injected anti-inflammatory medications, the potential benefits of anti-inflammatory medications delivered via iontophoresis far outweigh the minimal risk of a thermal injury due to electrical current.
Glenn D. Warden, MD, MBA, is a scientific advisor for a Salt Lake City-based company that specializes in transdermal drug delivery systems. A burn specialist, Warden has authored more than 600 manuscripts on burn care, and is the emeritus editor-in-chief for The Journal of Burn Care & Rehabilitation. He also served as the chief of staff of the Shriners Hospital for Children, Cincinnati Burns Hospital, and staff surgeon, Intermountain Shrine Hospital for Children, Salt Lake City. For more information, contact .
BIBLIOGRAPHY
Allen LV. Dexamethasone iontophoresis. US Pharmacist. November 1992:86.
Banga AK. Electrically-assisted Transdermal and Topical Drug Delivery. Bristol, Pa: Taylor & Francis Inc; 1998.
Hunt JL, Mason AD, Masterson TS, Pruitt BA. The pathophysiology of acute electric burns. J Trauma. 1976;16:335.
Safety by design. Available at: www.activatekinc.com/technology.html. Accessed January 17, 2007.
Saggini R, Zoppi M, Vecchiet F, Gatteschi L, Obletter G, Giamberardino M. Comparison of electromotive drug administration with ketorolac or with placebo in patients with pain from rheumatic disease: a double-masked study. Clin Ther. 1996;18:1169-1174.
Singh J, Roberts MS. Transdermal delivery of drugs by iontophoresis: a review. Drug Design Deliv. 1989;4:1-12.
Singh R, Roberts MS. Iontophoretic transdermal delivery of salicyclic acid and lidocaine to local subcutaneous structures. J Pharm Sci. 1993;82:127-131.
Smutok MA, Mayo MF, Gabaree CL, Ferslew KE, Panus PC. Failure to detect dexamethasone phosphate in the local venous blood postcathodic iontophoresis in humans. J Orthop Sports Phys Ther. 2002;32:461-8.





