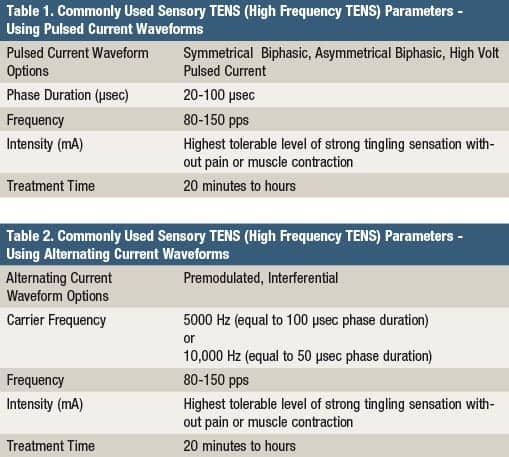
(Photo above) Therapist applies electrical stimulation to the anterior-posterior shoulder to treat pain associated with adhesive capsulitis using a clinical line-powered device.
By Joseph A. Gallo, DSc, AT, PT, and Kevin J. Silva, MS, AT
The clinical application of Transcutaneous Electrical Nerve Stimulation (TENS), as we know it today, began in 1967 by American neurosurgeon Norman Shealy. Based on Melzak and Wall’s gate control theory of pain modulation that was introduced in 1965, Shealy began to develop a surgically implanted dorsal column stimulator to treat patients who were experiencing chronic recalcitrant pain.1,2 Shealy used portable TENS devices to predict if patients with chronic pain would be good candidates for a surgically implanted dorsal column stimulator to manage their pain. The trials demonstrated that a large percentage of patients reported effective pain relief with TENS and requested to continue using TENS rather than have a dorsal column stimulator surgically implanted into their spine.1 Since then, TENS has become a common therapeutic intervention to reduce both acute and chronic pain. Most recently, over-the-counter (OTC) TENS devices have been made available to the general public in the retail marketplace.
Contemporary Use of TENS
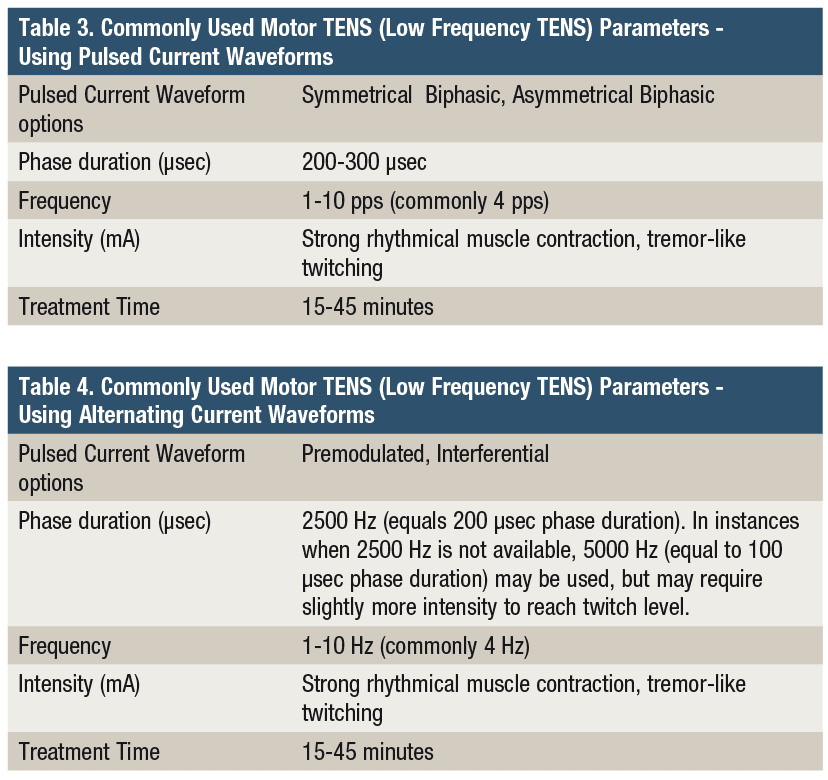
TENS is an inexpensive non-pharmacological method of treating acute and chronic pain without adverse side effects. For patients who are taking pain medication, the addition of properly dosed TENS to their pain management program can decrease their need for pain medication by 36% to 51%.3,4 The existing TENS clinical literature demonstrates conflicting evidence, which both supports and questions the effectiveness of TENS as a therapeutic intervention to treat pain. Despite overall mixed results in the pain management literature, “TENS has been shown to be effective for osteoarthritis, fibromyalgia, and neuropathic pain, all conditions with enhanced excitability and reduced inhibition.”5 Conflicting evidence within the existing TENS literature has led to more focused research and analysis to evaluate key factors regarding the dose response relationship and best practices for pain management. Clinically, the two most widely used TENS approaches are Sensory TENS (High Frequency TENS), and Motor TENS (Low Frequency TENS).
Sensory TENS (High Frequency TENS)
Sensory TENS is indicated for the treatment of acute pain and is characterized by the perception of a strong tingling sensation that is below the patient’s motor and pain threshold. The relatively gentle nature of Sensory TENS is less likely to violate tissue healing constraints in acute conditions. The primary objective of the intervention is to produce maximal depolarization of A-beta sensory nerve fibers which activate the spinal gating mechanism described by Melzack and Wall.2,4 A secondary endogenous opiate response induced by Sensory TENS has been long theorized. More recent research has validated this Sensory TENS induced endogenous opioid response for pain modulation.6 Sensory TENS (50-150 Hz) has been shown to activate delta-opioid receptors that supplement the spinal gating mechanism in modulating pain.6 It is important to note that research has also shown that caffeine can block the analgesic effect of Sensory TENS.7 Given that the half-life of caffeine is 4-6 hours, it is recommended that caffeine intake be limited and that it not be ingested in the 6 hours prior to TENS treatment.5
In order to maximally activate the wide diameter, heavily myelinated, and fast conducting A-beta sensory nerve fibers, a high pulse frequency and a short phase duration is used (Table 1). The intensity should be set to the desired physiologic response rather than a set number of milliamps. Intensity has been shown to be a critical parameter of TENS.5 In order to produce the strong, yet non-painful, sensory nerve depolarization characteristic of Sensory TENS, the stimulation can be turned up until the first signs of perceptible muscle twitching and then backed off slightly to the desired maximally strong tingling sensation.
The quick onset of electroanalgesia produced by Sensory TENS makes it a valuable intervention to pair with other active interventions during a therapy session, or as part of a home treatment program. Clinically, enhanced pain reduction and improved function is noted when TENS is combined with movement.8 The duration of TENS treatment does not need to be limited to 10 to 20 minutes, which has become common in clinical practice. The importance of home devices should not be underestimated. It affords the patient the ability to administer the treatment more frequently and for longer treatment durations, which may be necessary to keep the patient’s pain under control. It also allows them to increase the intensity (mA) throughout the treatment as accommodation to the stimulation occurs. This upward titration of the intensity throughout the treatment has been shown to be a key factor in enhancing analgesia.9
The upward titration of Sensory TENS during the treatment session should proceed to maximum sensory nerve stimulation, without motor nerve activation, and should be just below the pain threshold. In instances where muscle recruitment is interfering with achieving maximum sensory nerve depolarization, the phase duration can be shortened to the lower end of the recommended range (eg, 20-50 µsec) to further isolate sensory nerve recruitment. When using alternating current waveforms (Interferential or Premodulated), in devices that allow for the manipulation of carrier frequency, choosing a carrier frequency between 5,000 and 10,000 Hz will result in a shorter phase duration which is believed to be more effective in preferentially targeting the recruitment of the A-beta sensory nerves (Table 2).
Motor TENS (Low Frequency TENS)
Motor TENS is indicated for the treatment of subacute and chronic pain. It is characterized by the perception of a strong, yet tolerable, rhythmical muscle contraction which the patient perceives as muscle twitching. The activation of A-delta and A-alpha fibers during this treatment activates the release of endogenous opioids from the brain (periaqueductal gray and ventromedial medulla) into the blood and cerebrospinal fluid. Since the opioids travel down the spinal cord to the dorsal horn, where they chemically block pain, this mechanism of pain relief is also referred to as the descending endogenous opioid model. The longer-acting pain relief associated with Motor TENS (1-10 HZ) is attributed to the specific activation of mu-opioid receptors.5
Motor TENS is more aggressive than Sensory TENS, and provides longer-lasting pain relief than Sensory TENS.10 In order to activate the A-delta small diameter myelinated pain fibers, a low frequency between 1 to 10 pps (commonly 4 pps) and a phase duration of between 200 to 300 µsec is used. Intensity of stimulation has been shown to be an important factor in producing maximum analgesia. The intensity should be set to a strong visible, rhythmical, tremor-like muscle twitch. This strong rhythmical muscle twitch at a low frequency of stimulation produces longer-acting analgesia compared to Sensory TENS. However, it has greater potential to disrupt tissue healing when used in regions of acute conditions. Therefore, in acute pain and early stages of tissue healing, Sensory TENS is recommended.10-12 In instances where tissue healing has progressed, and pain is more chronic in nature, Motor TENS is indicated.11,13 As with Sensory TENS, the intensity (mA) should be titrated upward from time to time throughout the treatment session as habituation to the stimulus occurs. This makes a portable device a good choice because it is convenient and easily controlled by the patient. In the instance where a patient has developed a tolerance to opioid analgesics it is best to use Sensory TENS, since opioid users have a decreased response to motor TENS.14
Clinical Implications
Using sufficient intensity (mA) of electrical current during TENS applications can influence the magnitude of the analgesic response. Care must be taken to dose the current based on physiologic criteria and patient feedback. A more comfortable intensity of current does not necessarily correlate a better analgesic response.3 Conversely, “stronger” tingling sensation or a “stronger” muscle twitch is more likely to yield greater analgesia than a “comfortable” tingle or a “comfortable” twitch.3 Therefore, adequate initial dosing of the intensity at the beginning of each treatment and ongoing upward titration of intensity throughout the treatment, as accommodation occurs, are important clinical implications from the existing TENS literature.
Clinically, the magnitude and duration of Sensory TENS induced analgesia is typically not as great as Motor TENS induced analgesia.12,15,16 This may be explained by the fact that Sensory TENS and Motor TENS activate different opioid receptors. Sensory TENS (50-150 Hz) activates delta-opioid receptors, while Motor TENS (1-10 HZ) activates mu-opioid receptors.5 In the past, it was postulated that Motor TENS induced analgesia lasts up to 4 to 5 hours after 20 to 30 minutes of treatment based on the half-life of the endogenous opioid release being approximately 4.5 hours.10 More recent research suggests that analgesia induced by Motor TENS is longer lasting, and may last between 4 to 9 hours post treatment.10,15
In a recent study, it was reported that a 15-minute Motor TENS treatment produced up to 9 hours of analgesia post-treatment in 11 chronic low back pain patients.15 Further research on magnitude and duration of TENS induced analgesia is needed. The mechanisms of action and the type of opioid receptor activated by TENS are clinically relevant. For example, patients that have developed a tolerance to opioid medications do not experience pain reduction from Motor TENS (activates mu-opioid receptors), but do from Sensory level TENS (activates gating mechanism and delta-opioid receptors).14,17 It is important to note that opioid pain medications use the same mu-opioid receptors that Motor TENS activates to produce an electrically induced endogenous opioid response. Therefore, when tolerance through repeated opioid medication use develops, Motor TENS will not be effective.14
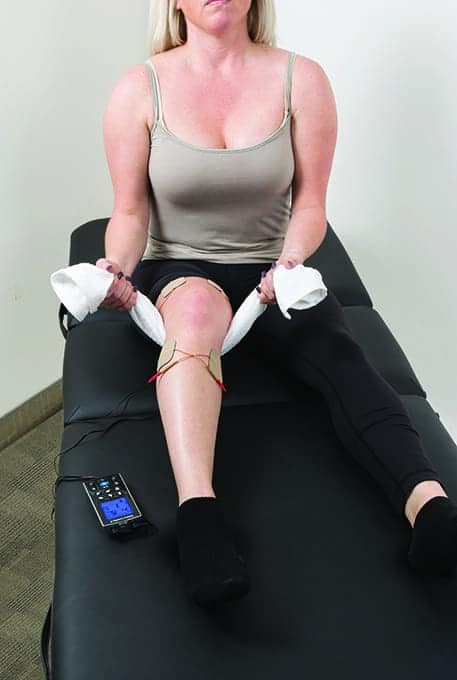
Portable TENS device in use to deliver sensory TENS for acute postsurgical pain, while performing heel slides to enhance knee range of motion.
Summary
TENS is an inexpensive non-pharmacological method of pain management that does not produce adverse side effects or the potential for addiction associated with opioid medications. In instances when opioid pain medication is prescribed, the adjunctive use of Sensory TENS can reduce the amount of pain medication necessary and often lead to earlier discontinuation of the opioid medication.3,4 Adequate dosing of TENS is essential in both clinical practice and the design of future research studies. Both pulsed and alternating current waveforms can be used to deliver Sensory and Motor TENS, providing the parameters and dosage are adjusted accordingly. TENS does not have to be a passive treatment. In fact, there is growing consensus that TENS is often more effective in decreasing pain during movement, functional activity, and therapeutic exercise interventions.8
There is currently a shift in the TENS literature towards analyzing previous studies to determine if key factors related to the dosing of TENS and the best practices related to study design have been employed. This approach has been helpful in identifying critical factors related to dosing and study design. This knowledge translates into better pain management outcomes in clinical practice and better study design in future TENS studies. RM
Joseph A. Gallo, DSc, AT, PT, is a professor and director of the athletic training program at Salem State University, Salem, Mass.
Kevin J. Silva, MS, AT, is the program assistant and adjunct faculty for the athletic training program at Salem State University, Salem, Mass. For more information, contact [email protected].
References
1. Shealy NC. Six years’ experience with electrical nerve stimulation for pain control. Adv Neurol. 1974;4:775-782.
2. Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979.
3. Bjordal JM, Johnson MI, Ljunggreen AE. Transcutaneous electrical stimulation (TENS) can reduce postoperative analgesic consumption. A meta-analysis with assessment of optimal treatment parameters for postoperative pain. Eur J Pain. 2003;7:181-188.
4. Van Der Ark GD, McGrath KA. Transcutaneous electrical stimulation in treatment of post-operative pain. AM J Surg. 1975;130(3):338-340.
5. Sluka KA, Bjordal JM, Marchand S, Rakel BA. What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature. Phys Ther. 2013; 93(10):1397–1402.
6. Leonard G, Goffaux P, Marchand S. Deciphering the role of endogenous opiods in high-frequency TENS using low and high doses of naloxone. Pain. 2010;151:215-219.
7. Marchand S, Li J, Charest J. Effects of caffeine on analgesia from transcutaneous electrical nerve stimulation. N Engl J Med. 1995;333:325–326.
8. Rakel B, et al. Transcutaneous electrical nerve stimulation (TENS) for control of pain during rehabilitation following total knee arthroplasty (TKA): A randomized, blinded, placebo-controlled trial. Pain. 2014:155(12);2599-2611.
9. Pantaleao MA, Laurino MF, Gallego NL, et al. Adjusting pulse amplitude during transcutaneous electrical nerve stimulation (TENS) application produces greater hypoalgesia. J Pain. 2011;12:581-590.
10. Cameron MH. Physical Agents in Rehabilitation: From Research to Practice. 4th ed. Elsevier-Saunders: St. Louis, MO; 2013.
11. Denegar CR, Saliba E, Saliba S. Therapeutic Modalities for Musculoskeletal Injuries. 4th ed. Champaign IL: Human Kinetics; 2016.
12. Starkey C. Therapeutic Modalities. 4th ed. FA Davis Company: Philadelphia, PA; 2013.
13. Knight KL, Draper DO. Therapeutic Modalities: The Art and Science. 2nd ed. Lippincott Williams & Wilkins: Baltimore, MD; 2013.
14. Leonard G, Cloutier C, Marchand S. Reduced analgesic effect of acupuncture-like TENS but not conventional TENS in opioid-treated patients. J Pain. 2011;12(2):213-221.
15. Tousigant-Laflamme Y, Laroche C, Beauileu C, Bouchard AJ, Boucher S, Michaud-Letourneau M. A randomized trial to determine the duration of analgesia following a 15- and a 30-minute application of acupuncture-like TENS on patient with chronic low back pain. Physiother Theory Pract. 2017:5;1-9.
16. Bracciano AG. Physical Agent Modalities: Theory and Application for Occupational Therapist. 2nd ed. Slack Inc: Thorofare, NJ; 2008.
17. Chandran P, Sluka KA. Development of opioid tolerance with repeated transcutaneous electrical nerve stimulation administration. Pain. 2003;102:195–201.